Tyrosine phosphatase biosensor as well as detection method and application thereof
A technology of tyrosine phosphatase and biosensor, which is applied in the field of analysis and detection, can solve the problems of poor specificity and low sensitivity, and achieve the effect of good specificity, high sensitivity and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
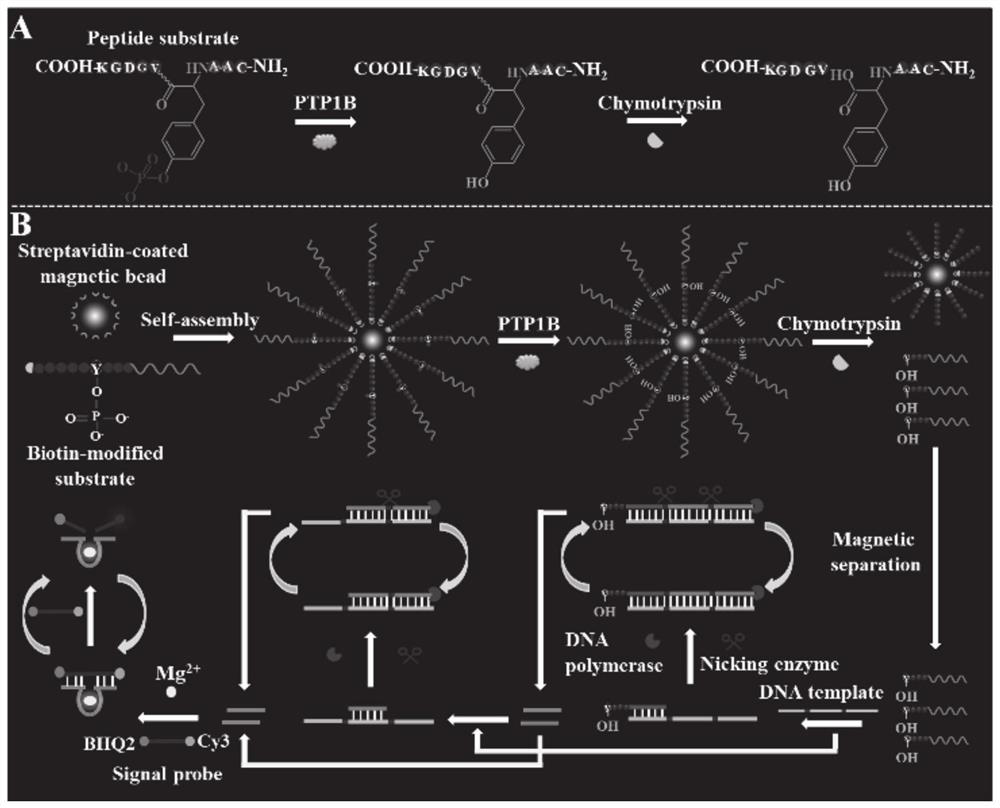
[0030] A typical embodiment of the present invention provides a tyrosine phosphatase biosensor, including magnetic beads, peptide-DNA conjugates, DNA templates, and signal probes;
[0031] The peptide-DNA conjugate includes a peptide chain and a first DNA sequence, one end of the peptide chain is connected to the 5' end of the first DNA sequence, and the peptide chain contains phosphorylated tyrosine; the other end of the peptide chain is set to be used for connecting a magnetic beads;
[0032]The DNA template is composed of two second DNA sequences and a third DNA sequence from the 5' end to the 3' end, the third DNA sequence is complementary to the first DNA sequence, and after the DNA template is hybridized with the first DNA sequence and then polymerized capable of forming double-stranded DNA with two nickase recognition sites, the complementary strand of the second DNA sequence being a deoxyribozyme;
[0033] The signal probe is a nucleotide sequence, the two ends of the...
Embodiment
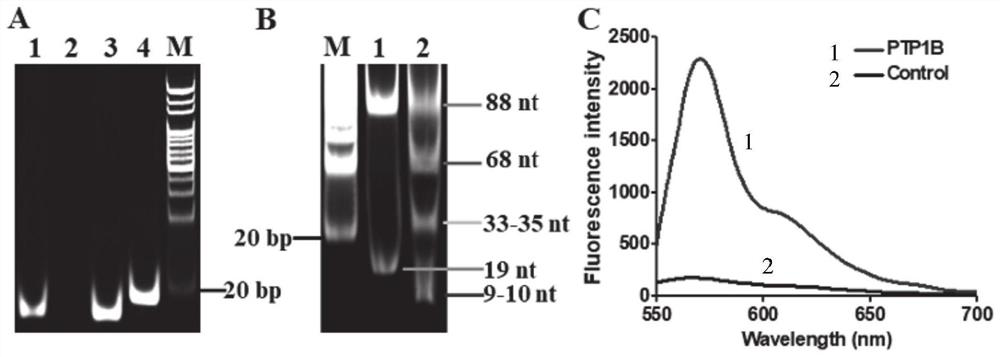
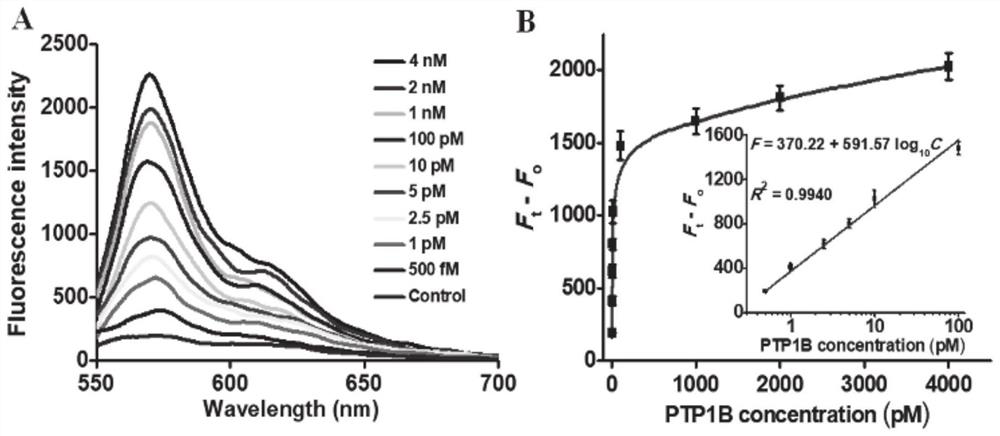
[0058] The connection reaction of the substrate and the magnetic beads coated with streptavidin: The connection reaction of the substrate and the magnetic beads (MBs) was carried out according to the instructions of Invitrogen Company. Add 200 μl of streptavidin-coated magnetic beads (MBs) solution (10 mg / ml) to the centrifuge tube and wash twice with 1× phosphate buffered saline (PBS) (pH 7.4). The supernatant was removed by magnetic separation and the magnetic beads (MBs) were resuspended to 200 μl. Then, 2 μl of peptide-DNA conjugates (100 μmol per liter) and 20 μl of 1×PBS were mixed with 128 μl of washed magnetic beads (MBs) solution, and the mixture was placed on a mixer at Incubate at room temperature for 30 minutes. The mixture was subjected to magnetic separation to remove unbound substrate and washed 5 times with 1X phosphate buffered saline (PBS). Then, the mixture was resuspended with 1× phosphate buffered saline (PBS) to a total volume of 80 microliters, and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com