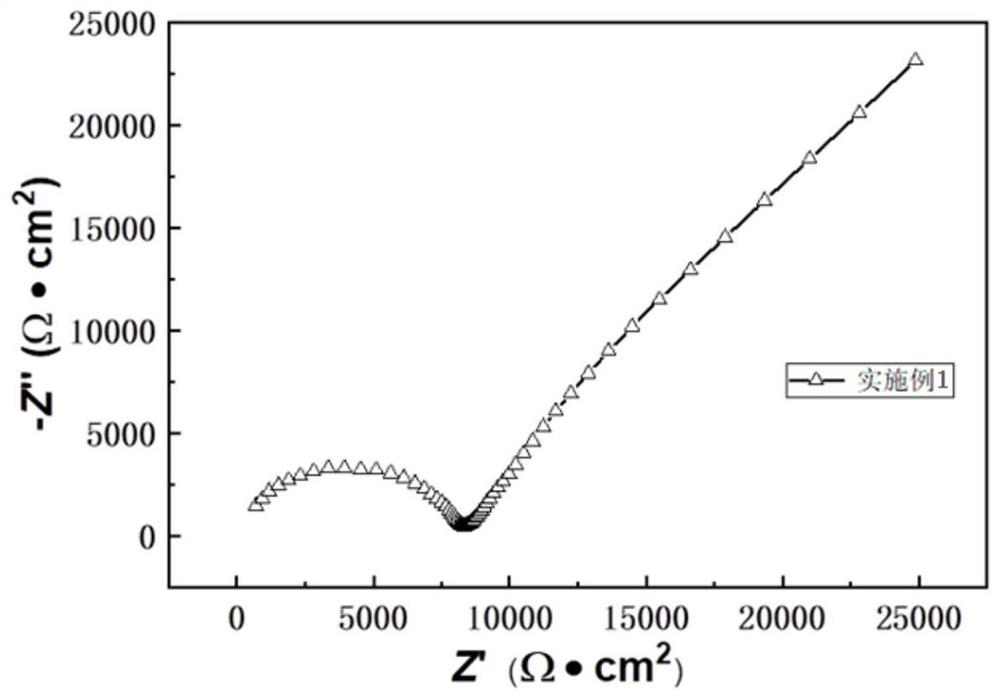
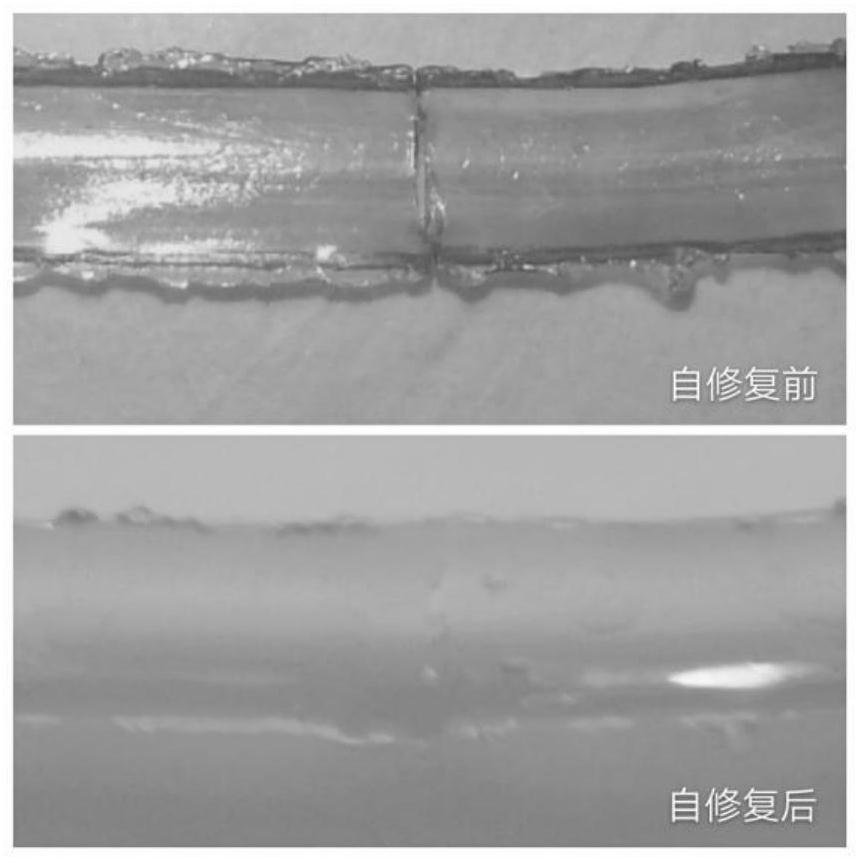
Imidazole polyion liquid electrolyte and preparation method and application thereof
An ionic liquid, imidazolium ion technology, applied in the field of imidazole polyionic liquid electrolyte and preparation thereof, can solve the problems of reduced ionic conductivity of electrolyte, poor high temperature safety, etc., and achieves improved high temperature safety, enhanced transmission efficiency, The effect of excellent self-healing and mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Polyionic Liquid Electrolyte Containing Butyl Acrylate Group.
[0029] Component one:
[0030] Weigh 1.88g 1-vinylimidazole and 3.14g 1-chloropropane in a 50mL round-bottomed flask, then add 8.00g anhydrous toluene, place in a 50°C oil bath and stir for 6h to obtain (1-propyl-3- Vinylimidazole) chloride, add 2.56g butyl acrylate monomer, add simultaneously the initiator (azobisisobutyronitrile) that is 2% with the total mass ratio of 1-vinylimidazole and butyl acrylate monomer, continue to react for 48h , a precipitate appeared, and the gel product obtained after vacuum filtration was extracted with anhydrous ether at 50°C for 48h.
[0031] The structural formula of (1-propyl-3-vinylimidazole) chlorine monomer is as follows:
[0032]
[0033] Wherein, n is any integer in 1-9.
[0034] The structural formula of butyl acrylate monomer is as follows:
[0035]
[0036] Add an ethanol solution of bis(trifluoromethylsulfonyl)imide lithium to carry out ani...
Embodiment 2
[0044] Example 2: Polyionic liquid electrolyte containing isodecyl acrylate group.
[0045] Component one:
[0046] Weigh 1.88g of 1-vinylimidazole and 3.14g of 1-chloropropane in a 50mL round bottom flask, then add 8.00g of anhydrous toluene, stir in an oil bath at 50°C for 6h, add 4.25g of isodecyl acrylate monomer , while adding an initiator (azobisisobutyronitrile) with a total mass ratio of 2% to 1-vinylimidazole and isodecyl acrylate monomer, the reaction was continued for 48 hours, a precipitate appeared, and the gel product obtained after vacuum filtration Extract with anhydrous diethyl ether at 50°C for 48h.
[0047] The structural formula of isodecyl acrylate monomer is as follows:
[0048]
[0049] Add an ethanol solution of lithium bis(trifluoromethylsulfonyl)imide to carry out anion displacement reaction for 24 hours, after precipitation in the water phase, vacuum filter to obtain an imidazolium ionic liquid copolymer containing isodecyl acrylate group, its s...
Embodiment 3
[0056] Embodiment 3: Polyionic liquid electrolyte containing isooctyl acrylate group.
[0057] Component one:
[0058] Weigh 1.88g of 1-vinylimidazole and 3.14g of 1-chloropropane in a 50mL round bottom flask, then add 8.00g of anhydrous toluene, stir in an oil bath at 50°C for 6h, add 3.68g of isooctyl acrylate monomer , while adding an initiator (azobisisobutyronitrile) with a total mass ratio of 2% to 1-vinylimidazole and isooctyl acrylate monomer, the reaction was continued for 48 hours, a precipitate appeared, and the gel product obtained after vacuum filtration Extract with anhydrous diethyl ether at 50°C for 48h.
[0059] The structural formula of isooctyl acrylate monomer is as follows:
[0060]
[0061] Add an ethanol solution of bis(trifluoromethylsulfonyl)imide lithium to carry out anion displacement reaction for 24 hours, after precipitation in the water phase, vacuum filter to obtain an imidazolium ionic liquid copolymer containing isooctyl acrylate group, it...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com