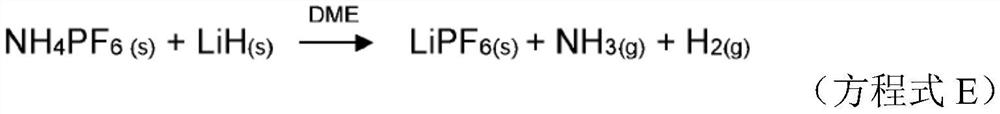
Production of lithium hexafluorophosphate
A technology of lithium hexafluorophosphate and perfluorocarbon, which is applied in the directions of lithium hexafluorophosphate, lithium compounds, electrochemical generators, etc., can solve the problems of expensive production time, time-consuming, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1: LiF and PF 5 Reactions of gases in the presence of cyclic or polycyclic perfluorocarbon solvents
[0083] A clean, thick-walled stainless steel reactor capable of handling gas pressures in excess of 10 bar was charged with 2 g of LiF solid powder from Sigma-Aldrich or Alpha-Aesar.
[0084] 60 ml of liquid perfluorodecalin (perfluorodecalin) was added to the reactor, so that the LiF was suspended in the perfluorodecalin.
[0085] The reactor was then sealed in a glove box and connected to a vacuum line, a high pressure indicator and a high pressure PF 5 On a system composed of gas cylinders.
[0086] will PF 5 Gas was introduced into the reactor from its feed bottle, thereby contacting the suspension of LiF in perfluorodecalin.
[0087] Continue to feed PF into the reactor 5 , until equilibrium is reached, maintaining equilibrium (PF 5 The air pressure rise was kept at 7 bar).
[0088] Allow the reaction to digest for at least 1 day.
[0089] Excess ...
Embodiment 2
[0097] Example 2: LiF and PF 5 Reactions of Gases in the Presence of Acyclic or Branched Perfluorocarbon Solvents
[0098] Solid form of LiF dispersed in liquid perfluoroheptane or range C 1 f 4 and C 6 f 14 to C 9 f 20 liquid in any acyclic perfluorocarbon.
[0099] The reaction that occurs is according to Reaction Equation 1:
[0100] The reaction temperature ranged from -94°C to 127°C.
[0101] The reaction pressure ranges from 0 kPa to 3000 kPa, more preferably up to 1000 kPa.
[0102] When will produce LiPF 6 dissolved in LiPF for solid form 6 When in a solvent, up to 99% LiPF can be achieved 6 Recovery rate, the solvent includes ethylene carbonate, propylene carbonate, dimethyl carbonate, dimethyl ether or any combination thereof.
Embodiment 3
[0103] Example 3: LiF and PF 5 Reactions of Gases in the Presence of Perfluorinated Aromatic Solvents
[0104] Dispersion of LiF in solid form in C 6 f 6 to C 10 f 8 In the range of liquid hexafluorobenzene or perfluorinated aromatic liquid compounds.
[0105] The reaction that occurs is according to Reaction Equation 1:
[0106] The reaction temperature ranges from 5°C to 100°C.
[0107] The reaction pressure ranges from 0 kPa to 3000 kPa, more preferably up to 1000 kPa.
[0108] When will produce LiPF 6 dissolved in LiPF for solid form 6 When in a solvent, up to 99% LiPF can be achieved 6 Recovery rate, the solvent includes ethylene carbonate, propylene carbonate, dimethyl carbonate, dimethyl ether or any combination thereof.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com