SARS-CoV-2 RBD Conjugated Nanoparticle Vaccine
A nanoparticle and vaccine technology, applied in the field of immunomedicine, can solve problems such as existing needs, and achieve the effects of easy production, high yield, and safe use of vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0090] The present invention also relates to a method for preparing the immunogenic complex as described above, comprising:
[0091] The immunogenic complex is obtained by expressing the fusion proteins of a) component and b) component, co-incubating after purification, and self-assembling.
[0092] According to yet another aspect of the present invention, it also relates to the application of the above-mentioned immunogenic complex, or the above-mentioned nanoparticle vaccine in the preparation of drugs for the treatment of novel coronavirus pneumonia.
[0093] The present invention further provides a method for protecting a subject from SARS-CoV-2 virus infection, which comprises administering to said animal an effective amount of the nanoparticle vaccine according to the present invention.
[0094] The subjects for the above purposes can refer to patients or animals suspected of carrying SARS-CoV-2, especially mammals, such as bats and civet cats; preferably primates, more ...
Embodiment 1
[0102] Embodiment 1. Design based on SARS-CoV-2 RBD nanoparticle vaccine
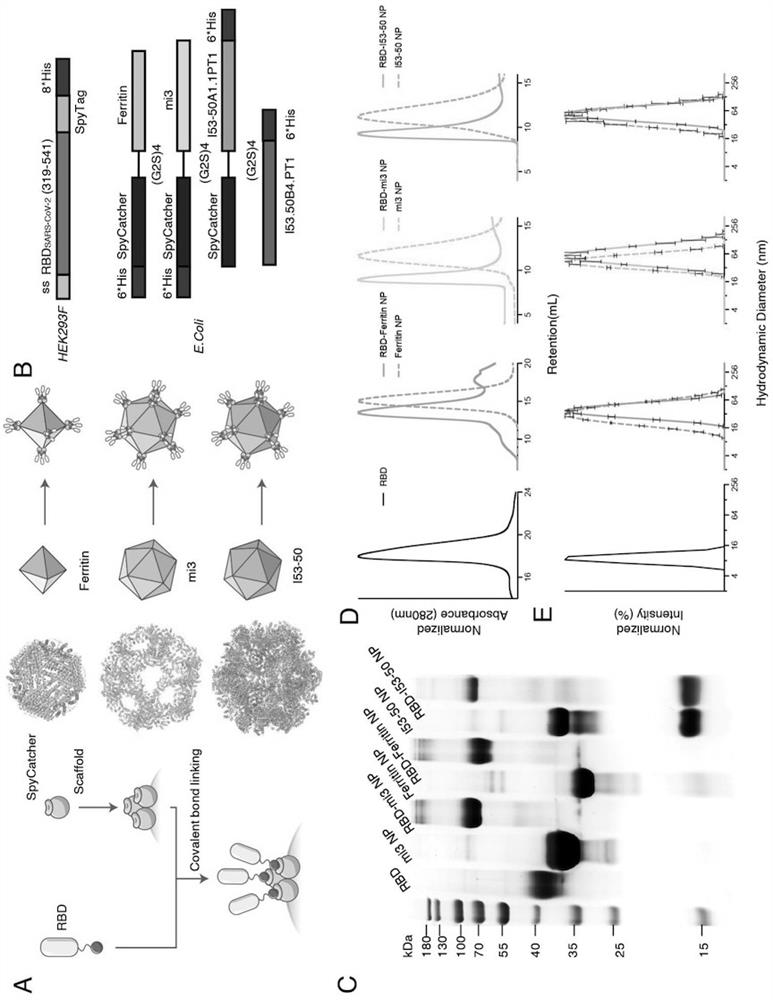
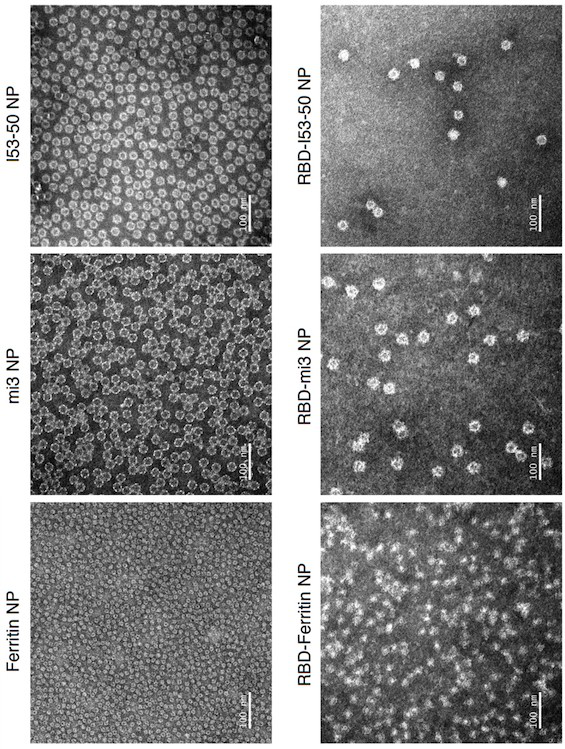
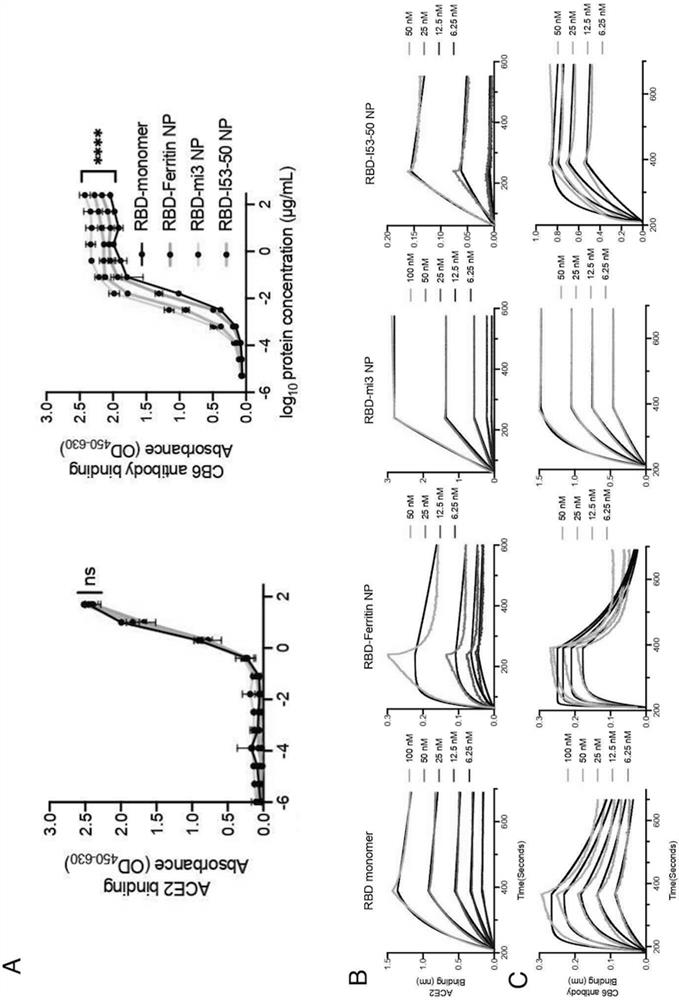
[0103] This example describes the design of Ferritin (24-mer), mi3 (60-mer) and I53-50 (120-mer) nanoparticles based on the SARS-CoV-2 RBD protein.
[0104] Considering that different nanoparticles can display different copy numbers of antigens on their surface, this example designs three SARS-CoV-2 RBD-conjugated nanoparticles: RBD-Ferritin, RBD-mi3 and RBD-I53-50.
[0105] Ferritin (SEQ ID NO: 2) is an octahedron composed of 24 subunits. Ferritin protein is the N-terminal 2-9 residues of the lower subunit of bullfrog (Rana catesbeiana) ferritin (UniProt: P07797) fused to 3-167 residues of Helicobacter pylori non-heme ferritin by molecular biological means. In order to eliminate the influence of the N-terminal potential glycosylation site, the present invention adopts N8Q and N19Q point mutations at residues 8 and 19 of bullfrog ferritin. To preserve the salt bridge between residues 6R and 14E of H. ...
Embodiment 2
[0109] Example 2. Expression and purification based on SARS-CoV-2 RBD nanoparticle vaccine
[0110] 1. Experimental materials
[0111] (1) Vectors and strains required for constructing recombinant vectors: mammalian expression vector VRC8400, Escherichia coli expression vector modified PET-28a+, Escherichia coli competent DH5a cells, Rosseta cells.
[0112] Protein expression cell line: HEK293-F cells (derived from human embryonic kidney epithelial cells).
[0113] (2) Reagents and consumables: PCR enzyme and recombinase (purchased from Novizyme Co., Ltd.), endonuclease (purchased from NEB), cell transfection reagent PEI-MAX (Polysciences, Inc., Cat. No. 24765- 1) Mammalian cell culture medium Union293 medium (purchased from Shanghai Yonglian Biology), histidine-tagged protein purification agarose magnetic beads (purchased from GE), and other conventional reagents and consumables are commercially available.
[0114] (3) Genes: △N1-SpyCatcher-Ferritin (SEQ ID NO: 18), △N1-Spy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com