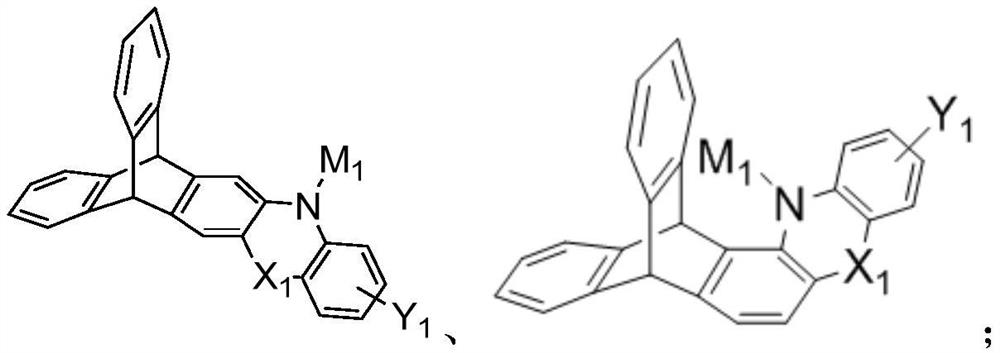
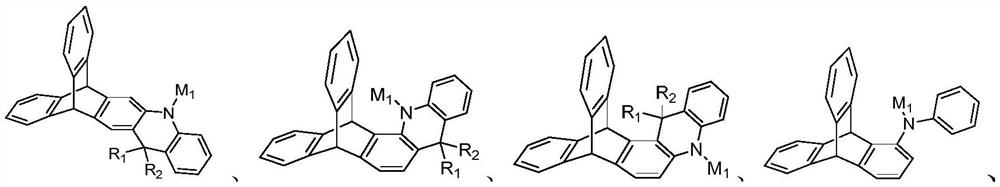
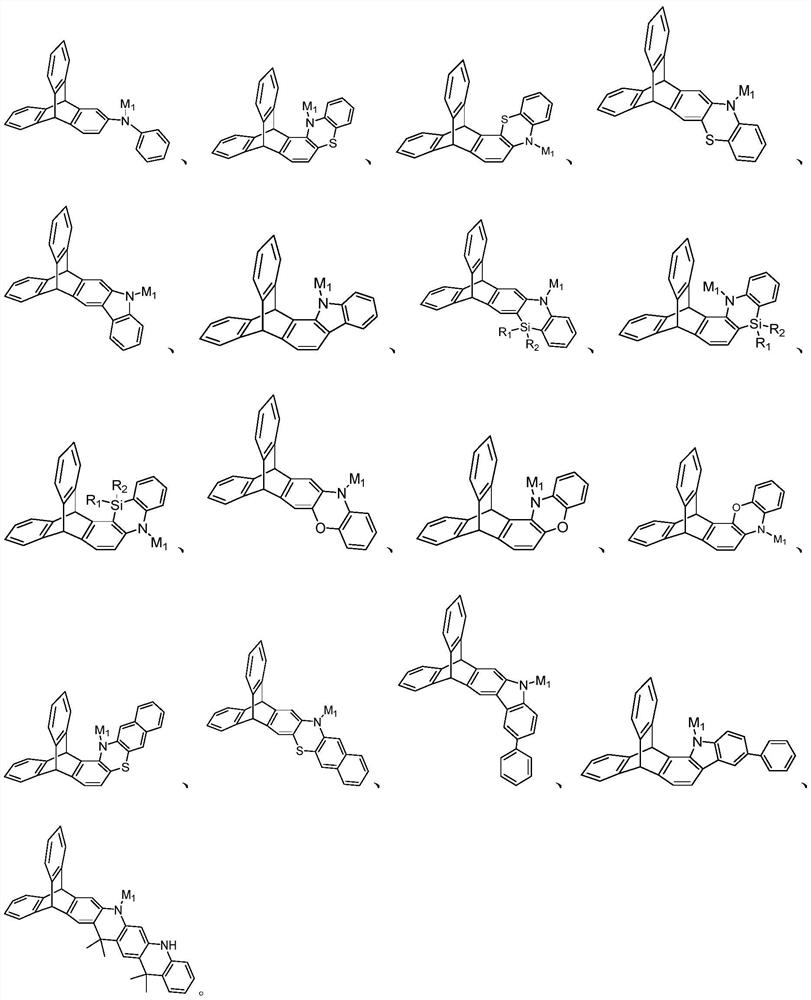
Triptycene D-A type thermal activation delayed fluorescence material, electronic device and application thereof
A technology of thermally activated delayed and fluorescent materials, applied in luminescent materials, electric solid devices, electrical components, etc., can solve problems including high cost, strong triplet annihilation, low blue emission stability, etc., and achieve good solubility, The effect of good thermal stability and easy regulation of molecular structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046]The structural formulas of the thermally activated delayed fluorescent materials TTC-DMAC-TRZ (compound 60) and TTC-DMAC-PM (compound 61) in this example are as follows:
[0047]
[0048] The specific synthesis process of the above thermally activated delayed fluorescent material is as follows:
[0049]
[0050] Preparation of Compound 2: Select a 100mL two-necked flask, add 1-aminotriptycene (2.36g, 10mmol), methyl anthranilate (3.62g, 24mmol), palladium acetate (224.5mg, 1mmol) under argon protection , cesium carbonate (13.0 g, 40 mmol), tri-tert-butylphosphonium tetrafluoroborate (754.3 mg, 2.6 mmol) and 30 mL of toluene were reacted at 120° C. for 12 hours. After cooling to room temperature, the inorganic salt was filtered through diatomaceous earth, and separated by silica gel column chromatography to obtain 2.86 g of white solid with a yield of 76%. MS(EI):m / z 430.1[M + ].
[0051]
[0052] Preparation of TTC-DMAC: Compound 2 (1.30 g, 3.4 mmol) was diss...
Embodiment 2
[0058] The preparation of the TTC-PXZ of the present embodiment, reaction formula is as follows:
[0059]
[0060] Preparation of Compound 2: Select a 250mL two-necked flask, add 2-hydroxytriptycene (1.60g, 5.9mmol) and 30mL toluene under the protection of argon, and slowly add N-bromosuccinimide (1.05g, 5.9mmol) , reacted at 0°C for 2 hours. After the reaction, it was separated by silica gel column chromatography to obtain 1.80 g of white solid with a yield of 90%. MS(EI):m / z 348.0[M + ].
[0061] Preparation of compound 3: Select a 100mL two-necked flask, add compound 2 (0.80g, 2.3mmol), o-fluoronitrobenzene (0.39g, 2.7mmol), potassium carbonate (0.63g, 4.6mmol) and 30mL under the protection of argon N,N-Dimethylformamide (DMF), reacted at 100°C for 12 hours. After cooling to room temperature, it was poured into water and filtered to obtain a solid, which was separated by silica gel column chromatography to obtain 0.86 g of a white solid with a yield of 78%. MS(EI):m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com