Copper (I) complex based on butanone and salicylaldehyde amino triazole Schiff base, and synthesis method thereof
A synthesis method and complex technology, applied to copper organic compounds, 1/11 group organic compounds without C-metal bonds, organic chemical methods, etc., can solve problems that have not been seen before, and achieve low cost and easy availability of raw materials , The effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Complex [Cu(HL) 2 The in-situ synthesis method of Cl] comprises the steps:
[0031] (1) Weigh 9.4 mg (0.05 mmol) of salicylaldehyde acetal-3-amino-1,2,4-triazole Schiff base and 5.0 mg (0.05 mmol) of CuCl at one end about 19 cm long with an inner diameter of 1.0 cm Into the sealed glass tube, add 2.0 mL methyl ethyl ketone, oscillate to make it fully mixed; then place the other end of the glass tube under vacuum to seal;
[0032] (2) Put the above-mentioned sealed glass tube in an oven at 120°C for reaction, stop heating after 72 hours of reaction, and then cool down to room temperature at 10°C per hour to obtain colorless long strip crystals, namely [Cu(HL) 2 Cl] product with a yield of 41.2%.
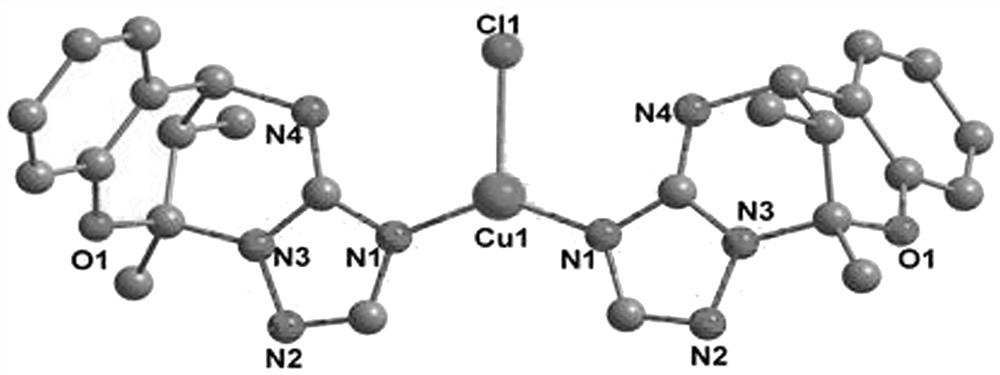
[0033] see figure 1 , it can be seen from the figure that there is only one crystallographically independent Cu(I) ion in the smallest unit of the complex of Example 1. Each N atom on the body (HL) is coordinated to form a chemical formula [Cu(HL) 2 Cl] complexes. The sm...
Embodiment 2
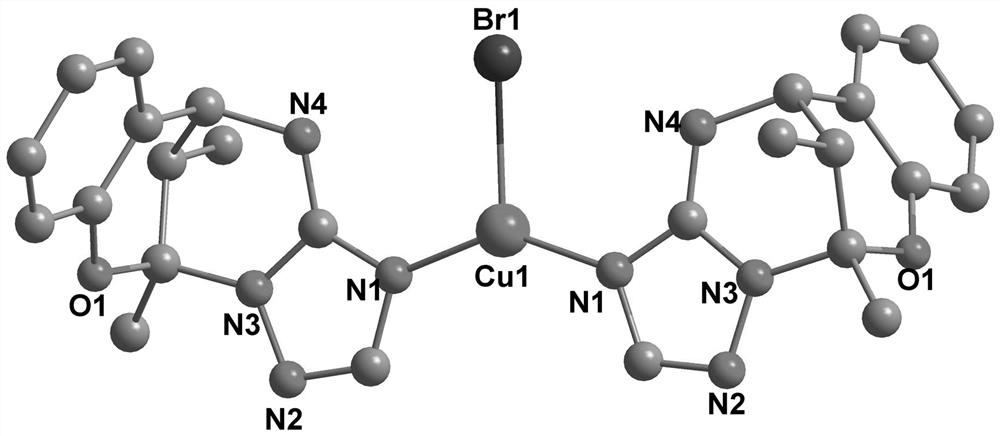
[0035] Complex [Cu(HL) 2 The in-situ synthesis method of Br] replaces CuCl in Example 1 with CuBr 7.2 mg (0.05 mmol), and other conditions remain unchanged, and also obtains colorless long strip crystals, i.e. [Cu(HL) 2 Br] product, productive rate is 40.2%, and molecular structure sees figure 2 , the structure is the same as figure 1 The only difference is that Cl atoms are replaced by Br atoms.
Embodiment 3
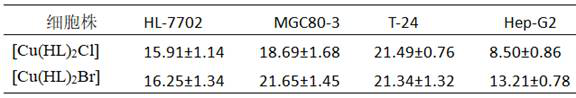
[0037] Determination of the IC of the two complexes of the examples to some cell lines by MTT colorimetry 50 The values are shown in Table 1, where HL-7702 is a normal human liver cell line, A549 is a human lung cancer cell line, T-24 is a human bladder cancer cell line, and MGC80-3 is a human gastric cancer cell line.
[0038] Table 1, the IC of the example complexes on different cell lines 50 Value (μM)
[0039]
[0040] The results in Table 1 show that the two complexes of the examples have certain antitumor activity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com