Preparation method of 3-aminoisoxazole
A technology of aminoisoxazole and hydroxyurea, applied in the field of medicine, can solve the problems of high cost, high process risk, easy filling and the like, and achieves the effects of improved yield, simple process steps, safe operation and environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
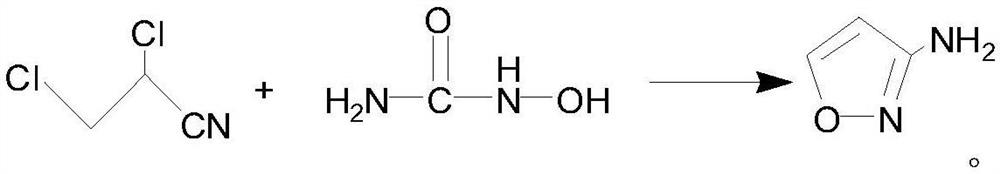
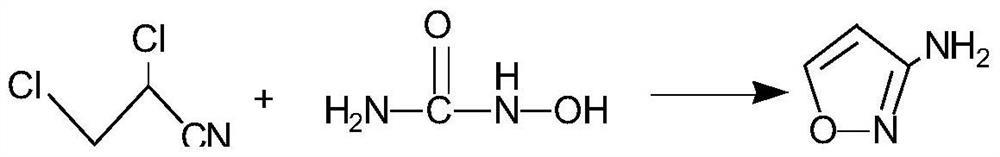
[0030] A preparation method of 3-aminoisoxazole, the reaction scheme is as follows:
[0031]
[0032] Specific steps are as follows:
[0033] Put liquid caustic soda, hydroxyurea, and N,N-dimethylformamide into the reaction kettle; add 2,3-dichloropropionitrile dropwise under temperature control at 10-20°C; after the dropwise addition, stir at 10-30°C for 4 hours. After the reaction, pass in carbon dioxide, adjust the pH to 8-9, and filter with suction; extract the mother liquor with methyl isobutyl ketone, dry the extract with anhydrous sodium sulfate, decolorize and filter it with activated carbon, and desolventize the extract to obtain a light yellow oil and then distilled under reduced pressure to obtain a colorless oil.
[0034] The molar ratio of hydroxyurea, 2,3-dichloropropionitrile and sodium hydroxide is 1:1:3~4.
[0035] The weight ratio of hydroxyurea to N,N-dimethylformamide is: 1:0.05-0.10.
[0036] After the dropwise addition of 2,3-dichloropropionitrile i...
Embodiment 1
[0039] Preparation of 2,3-dichloropropionitrile: put 130g of acrylonitrile into a 500ml reactor, start stirring; then put in 1.3g of N,N-dimethylformamide and 0.13g of pyridine in turn; cool the contents of the reaction bottle to 5~15°C, slowly feed chlorine gas into the reaction bottle, keep the reaction temperature not exceeding 20°C; weigh, the molar amount of the gas introduced is 1.05~1.1 times the molar amount of acrylonitrile, and the aeration is completed; the temperature is controlled at 15~25°C Stir for more than 5 hours.
[0040] Nitrogen blows out excess chlorine until the reaction solution is slightly yellow or colorless and transparent.
[0041] Preparation of 3-aminoisoxazole: put 600g of liquid caustic soda with a mass concentration of 20% into a 2L reactor, start stirring; then put in 76g of hydroxyurea and 7.6g of N,N-dimethylformamide in sequence, and cool down; 15°C Thereafter, 126.7 g of 2,3-dichloropropionitrile was started to be added dropwise; during t...
Embodiment 2
[0044] Preparation of 2,3-dichloropropionitrile: put 1.30kg of acrylonitrile into a 2000ml reactor, start stirring; then put in 13g of N,N-dimethylformamide and 1.3g of pyridine in turn; cool the contents of the reaction bottle to 5~15°C, slowly feed chlorine gas into the reaction bottle, keep the reaction temperature not exceeding 20°C; weigh, the molar amount of the gas introduced is 1.05~1.1 times the molar amount of acrylonitrile, and the aeration is completed; the temperature is controlled at 15~25°C Stir for more than 5 hours.
[0045] Preparation of 3-aminoisoxazole: put 6.0kg of liquid caustic soda with a mass concentration of 20% into a 20L reactor, start stirring; then put in 760g of hydroxyurea and 57g of N,N-dimethylformamide in sequence, and cool down; 15°C Next, start to add 1.27kg of 2,3-dichloropropionitrile dropwise; keep the temperature at 10-20°C during the dropwise addition, and the dropwise addition time is about 2 hours. After the dropwise addition, keep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com