A carbon catabolism regulatory protein CCPA mutant K31A
A technology for catabolism and regulation of proteins, which is applied in the field of carbon catabolism regulation protein CcpA mutant K31A, recombinant expression vectors and recombinant microbial cells, which can solve the problems of destroying bacterial metabolic pathways and restricted bacterial growth, so as to reduce preference sex, altering the effect of carbon catabolism repression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Choice of key amino acids of CCPA protein in Bacillus bacillus
[0040]The CCPA gene of the Jacket Bacillus is mainly combined by a global regulatory factor mainly by protein and a nucleic acid site, and then exerts its regulation function, the relative position of the sub-structure of the CCPA protein and the sub-structure of its corresponding binding site after the nucleic acid is changed. Amino acid acting in this transform process may be located at both ends of the α spiral in the CCPA protein sub-structure. At the same time, the CCPA protein and the nucleic acid are identified by the protein and the CRE site, and the amino acid in which the primary action thereof in the protein and the CRE site may be located in the middle of the α spiral, so we are mainly distributed at CCPA protein when selecting the amino acid sites. Α The two ends of the spiral and the middle.
Embodiment 2
[0041] Example 2 Construction of CCPA protein expression vector
[0042] The CCPA gene was amplified by the CCPA protein genome of the Jacket Bacillus, and the CCPA gene was amplified, and the resulting gene was attached to the PET28a vector, and the Bacillus CCPA protein expression vector was constructed. It was converted to E. coli BL21 (DE3) to construct recombinant strains.
Embodiment 3
[0043] Example 3 CCPA protein at CCPA protein and expression and purification
[0044] The CCPA protein expression vector constructed in Example 2 was a template to a fixed point mutation of the CCPA protein of the Jacket Bacillus underclothes.
[0045] The order of the fixed-point mutant primer is as follows:
[0046] K31A-F (SEQ ID No.5): 5'GaAatcggaacgtcgccccgcgcgagaagaagaaggtgcttgaagccc3 ';
[0047] K31A-R (SEQ ID No.6): 5'tctcgtcgggcgcgcgttcgggttccgttcacaaccctggAAACG3 '.
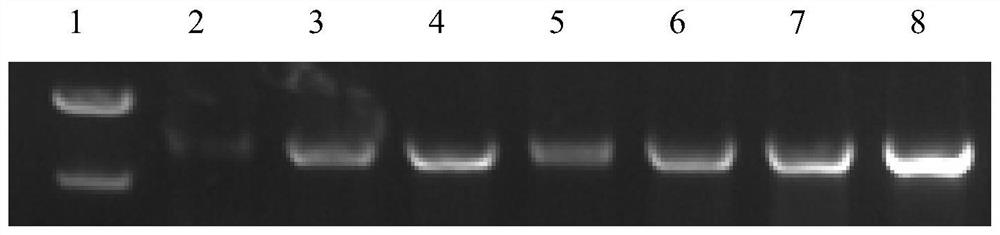
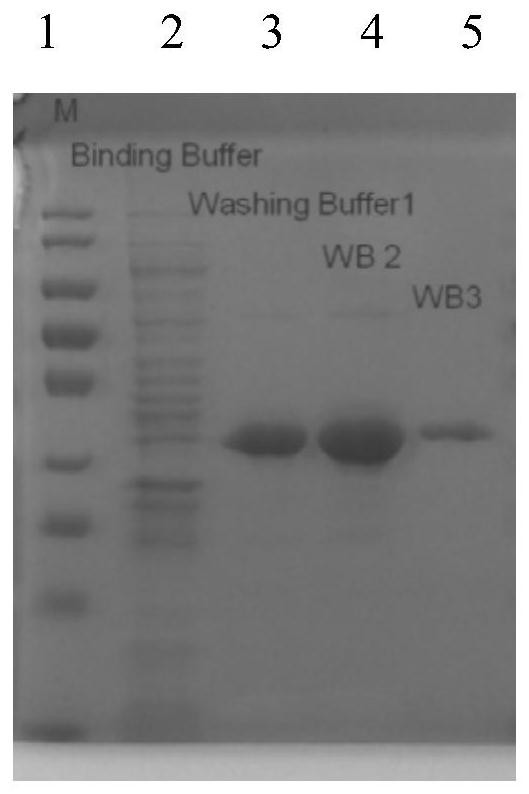
[0048] The CCPA protein after the fixed point mutation (amino acid sequence, such as SEQ ID NO.2, encoding the gene nucleotide sequence, such as SEQ ID NO.1), is subjected to heterologous expression and purification, colony PCR expansion Introduction figure 1 As shown, SDS-PAGE gel verification figure 2 As shown, it indicates that the CCPA protein prototype of the Jacket Bacillus CCPA protein is successfully introduced into E. coli and successfully expressed in E. coli.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com