Production process of high-purity novolac epoxy resin with ultralow phenolic hydroxyl group content
A technology of novolac epoxy resin and ultra-low phenolic hydroxyl group, which is applied in the production process of high-purity novolac epoxy resin, which can solve the problems of easy water absorption, unevenness, and low conversion rate, etc., and achieve light color and yellowing resistance. Good, high-purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Under acidic environment, phenol (o-cresol, bisphenol A) and formaldehyde generate novolac phenolic resin. In the industry, oxalic acid is basically used and added at one time as a catalyst. The present invention also uses oxalic acid, but adds it in stages instead.
Embodiment approach
[0033] 1) Synthesis of epoxy resin
[0034] S1.1: Prepare materials phenol 1000kg, formaldehyde 340kg and oxalic acid 2.7kg;
[0035] S1.2: Put phenol, formaldehyde, and 1.5kg oxalic acid into the reactor respectively, start to heat up to 100±2°C, perform a reflux for 2-5 hours, and then put 1.2kg oxalic acid into the reactor after the reflux, Under the condition of keeping the temperature constant, carry out secondary reflux for 2-5 hours;
[0036] S1.3: After the secondary confluence, start to recover phenol, by raising the temperature to 160°C, and vacuum 5kpa;
[0037] S1.4: After recovering phenol, get the phenolic resin, weigh it and prepare for the next process.
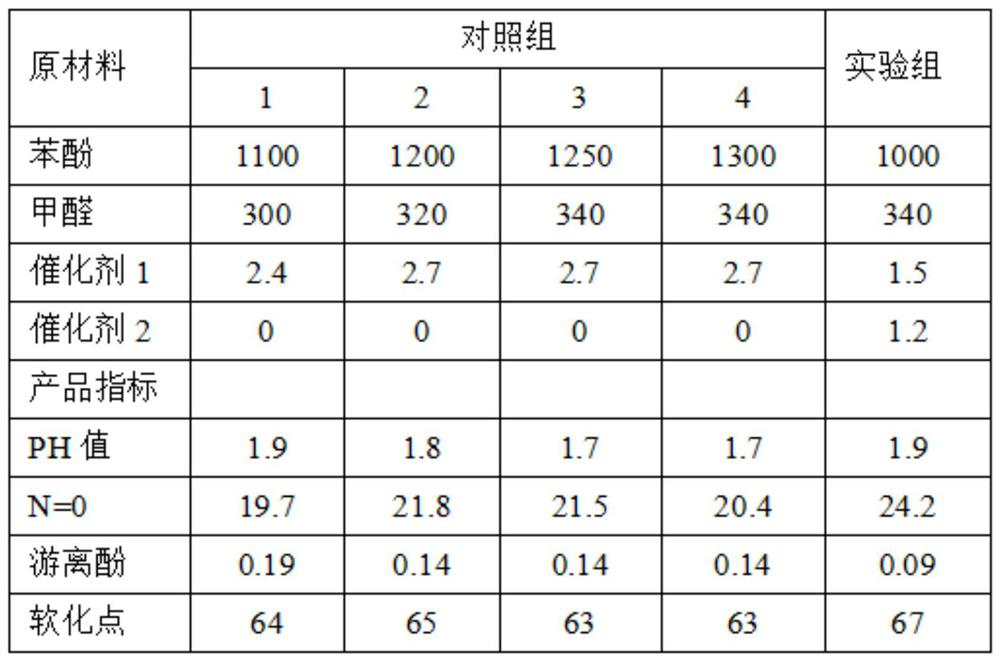
[0038] In the phenolic resin produced by this reaction method, the molecular content of n=0 is 20-25%, which is more suitable for the synthesis of epoxy resin.
[0039] see figure 1 ,
[0040] Among them, the pH value refers to the pH value of the system during the reaction process, which is measured by a...
Embodiment 2
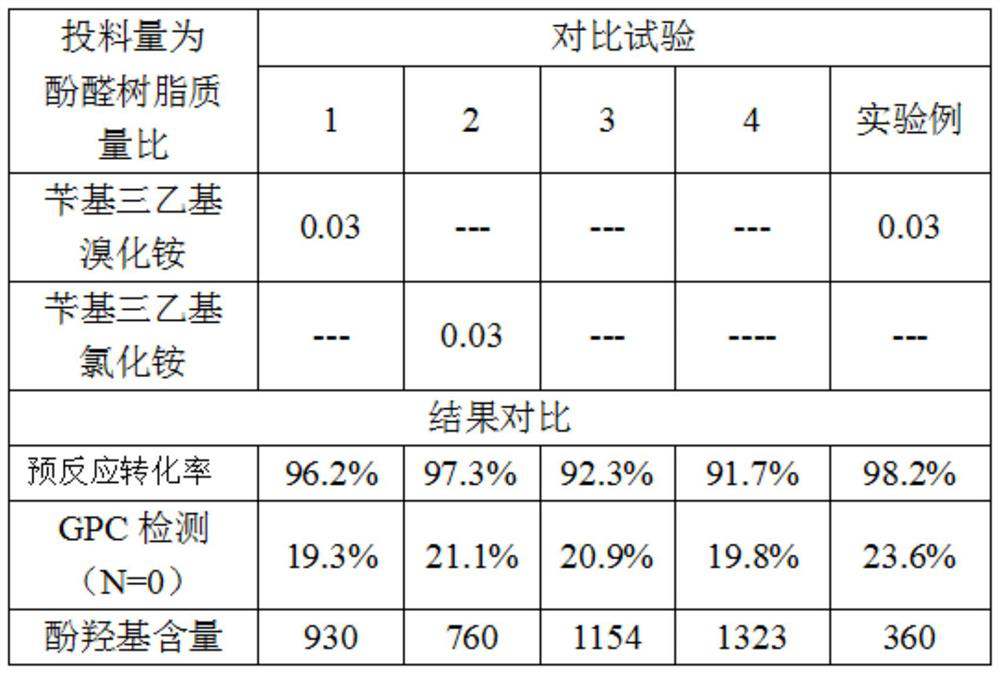
[0046] The conversion rate of phenolic resin and epichlorohydrin in the pre-reaction stage is the focus of affecting the content of phenolic hydroxyl groups. Manufacturers in the industry generally use liquid caustic soda as a catalyst to carry out the ring-opening reaction of phenolic resin and epichlorohydrin. The present invention uses Benzyltriethylammonium bromide and benzyltriethylammonium chloride are used as phase transfer catalysts to promote the catalysis of liquid caustic soda to achieve the purpose of improving the conversion rate at a lower reaction temperature.
[0047] The present invention provides another embodiment:
[0048] 2) Ring opening reaction
[0049] S2.1: Prepare benzyltriethylammonium bromide or benzyltriethylammonium chloride as a phase transfer catalyst;
[0050] S2.2: Put the synthesized epoxy resin into the reactor, add epichlorohydrin into the reactor, stir evenly, add the phase transfer catalyst, and adjust the temperature to 54±2°C;
[0051...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com