Aminodeoxychorismate synthase mutant t426i with altered enzyme activity and application thereof
A technology of chorismate and synthase, applied in the fields of genetic engineering and fermentation engineering, can solve problems such as influence level, and achieve the effect of good industrial application value and prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Point mutation of aminodeoxychorismate synthetase to T426I
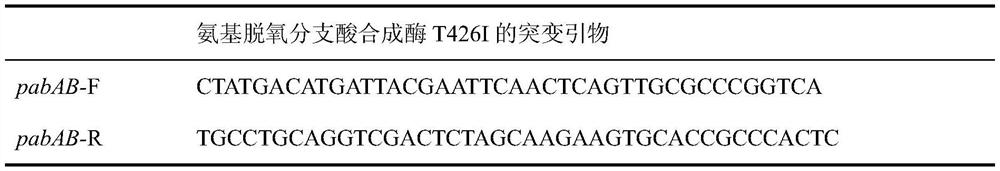
[0037] Site-directed mutagenesis was carried out by PCR method, saturation mutation was performed on the P426 site of ΔSSAAI aminodeoxychorismate synthetase, and the tyrosine at the 426th site was mutated into isoleucine. Using the strain genome as a template, select the 500 bp before and 500 bp after the point mutation as the amplification target, and use primers pabAB-F and pabAB-R to amplify the homology arm gene fragment containing the point mutation. It was ligated with the plasmid pK18mobsacB double-digested by EcoR I and Xba I to construct a reverse mutation plasmid, and electrotransformed Corynebacterium glutamicum; cultured at 30°C for 3 days, and the single colony grown was a recombinant colony. Pick a single colony and inoculate it into a seed liquid medium containing 50 μg / mL Kan, cultivate it at 30°C on a reciprocating shaker at 120 rpm, dilute it to 10% sucrose screening medium, and cu...
Embodiment 2
[0041] Example 2: Point mutation of aminodeoxychorismate synthetase to other amino acids
[0042] Site-directed mutagenesis was carried out by PCR method, saturation mutation was carried out on the P426 site of aminodeoxychorismate synthetase, and the tyrosine at the 426th site was mutated into other 19 kinds of amino acids. Subsequent other steps are the same as in Example 1. The primers used for T426 to carry out other non-T426I mutations are the same as SEQ ID NO.3 and SEQ ID NO.4.
Embodiment 1-2
[0043] Embodiment 1-2 result analysis
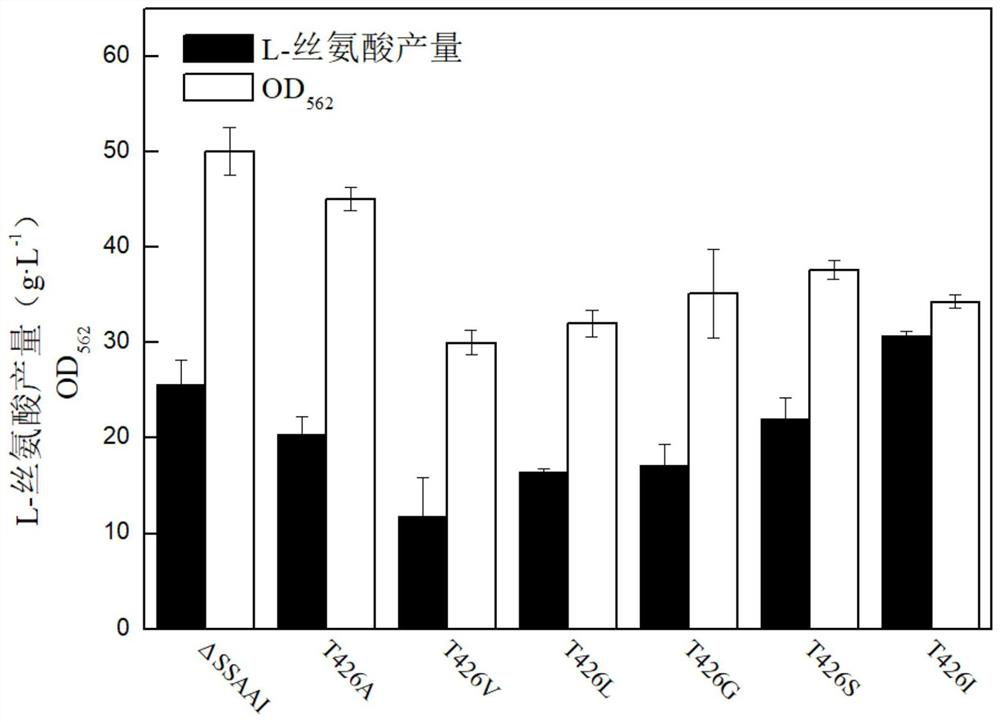
[0044] (1) The effect of point mutation on the activity of aminodeoxychorismate synthase
[0045] The obtained series of aminodeoxychorismate synthase mutant strains were tested for enzyme activity, and the effect of point mutations on the aminodeoxychorismate synthase activity was analyzed. The kit was used to measure the aminodeoxychorismate synthesis of the starting strain ΔSSAAI and the mutant strain T426I after 60 hours of cultivation. Enzyme specific enzyme activity.
[0046] The results are shown in Table 2. Taking the unmutated aminodeoxychorismate synthetase starting strain as a control group, the aminodeoxychorismate synthase obtained in Example 1 was point-mutated into T426I and the aminodeoxychorismate synthetase obtained in Example 2. The mutant strains with enzyme point mutations to other amino acids were used as the experimental group, and the specific enzyme activities of amino-deoxychorismate synthetase after cultivatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com