Complex nanoparticles of poloxamer and/or combination of poloxamer amine and PEG lipids
A technology of poloxamine and poloxamer, which is applied in the field of in vivo and in vitro cell gene transfection and cationic complex nanoparticles, which can solve the problems of low transfection efficiency, instability, and high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Preparation of various prescriptions of cationic complex nanoparticles LLLRNA1003
[0046] Prescription 1: The molar ratio of T304:PEG2000-C-DMG:DSPC:mPEG2000-C-CLS is 100:3:20:80
[0047] First take T304 out of the 4°C refrigerator and equilibrate to room temperature, weigh it at room temperature and add nuclease-free ultrapure water to dissolve it, shake it fully with a mediator for 5 minutes, and let it stand overnight to obtain stock solution A; mix PEG2000-C-DMG, DSPC and mPEG2000-C-CLS were taken out from the -20°C refrigerator, equilibrated to room temperature and opened, weighed at room temperature with a molar ratio of 3:20:80, and dissolved in chloroform in a round bottom flask. Use a rotary evaporator to evaporate and remove chloroform, pour the stock solution A into a round-bottomed flask, and continue to sonicate for 60 minutes at 40 degrees Celsius with a 2-second pause and a 2-second pause, transfer it to a dialysis bag with a MWCO of 5000, and...
Embodiment 2
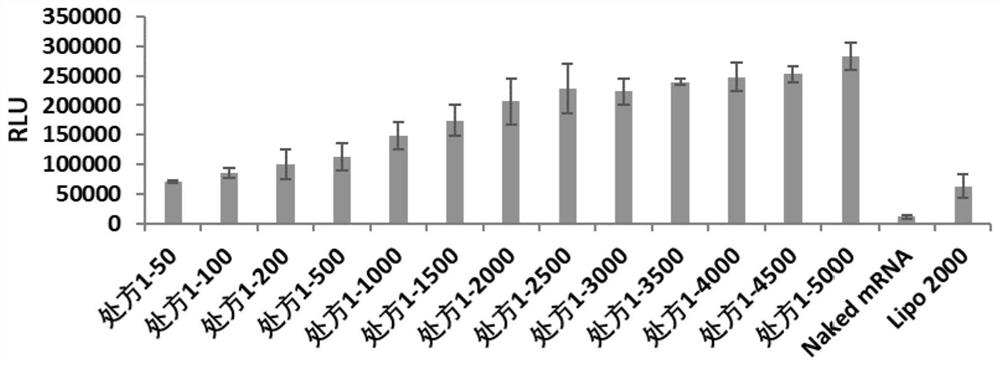
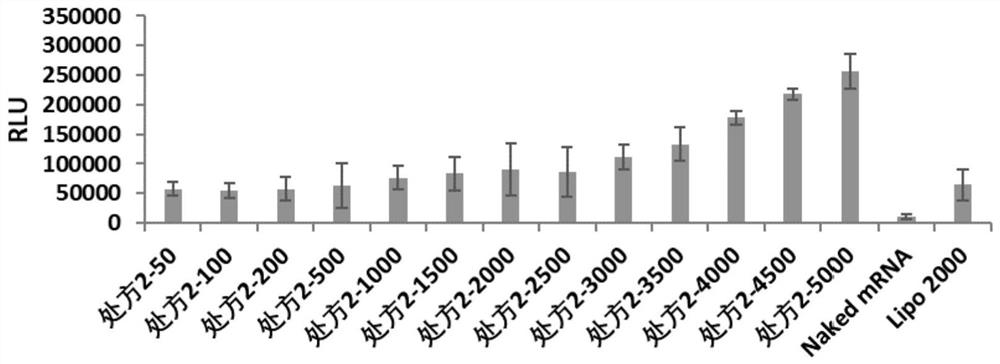
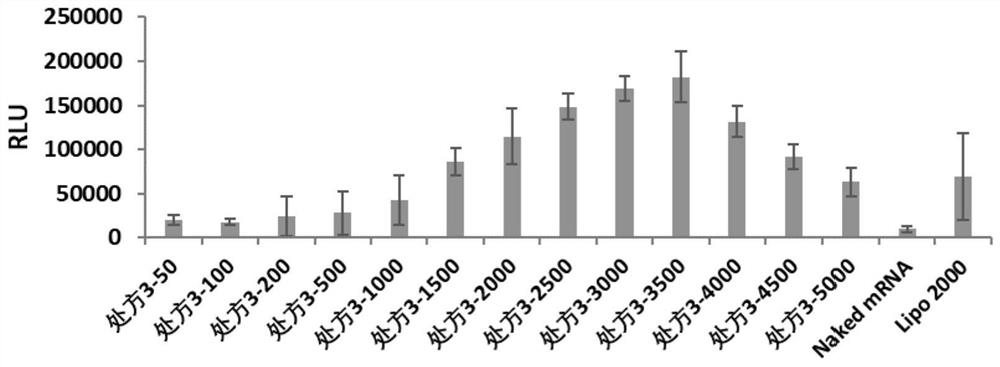
[0062] Example 2: Characterization of the cationic complex nanoparticle formulation of poloxamer and / or poloxamine combined with lipid
[0063] The present invention relates to the particle size and potential of nanoparticles. Malvem Zetasizer Nano ZSE is used to test the cationic complexes containing FLuc-mRNA in prescription 1, prescription 2, prescription 3, prescription 4, prescription 5, prescription 6, prescription 7 and prescription 8. granules were made into 1ml of the solution to be tested, and the effects of cationic complex nanoparticles without FLuc-mRNA were investigated under the condition of 25°C. The particle size (IntensityMean), surface potential (Zeta Potential) and polydispersity (PDI) of the dynamic light scattering nanoparticles are shown in Table 1.
[0064] According to the optimal mass ratio of nanoparticles and FLuc-mRNA in each prescription (the optimal mass ratio of prescription 1 is 5000, the optimal mass ratio of prescription 2 is 5000, the optima...
Embodiment 3
[0069] Example 3: Testing the loading efficiency of various formulations of poloxamer and / or poloxamine and lipid-combined cationic complex nanoparticles
[0070] Quant-iT RiboGreen RNA Detection Kit (ThermoFischer Company) was used to measure the inclusion of FLuc-mRNA by each prescription of LLLRNA-1003 (prescription 1, prescription 2, prescription 3, prescription 4, prescription 5, prescription 6, prescription 7 and prescription 8). Sealing rate, the specific method refers to the kit instructions, the brief processing method of the present invention is:
[0071]According to the optimal mass ratio of nanoparticles and FLuc-mRNA in each prescription (the optimal mass ratio of prescription 1 is 5000, the optimal mass ratio of prescription 2 is 5000, the optimal mass ratio of prescription 3 is 3500, the optimal mass ratio of prescription 4 is 100, The optimal mass ratio of prescription 5 is 1500, the optimal mass ratio of prescription 6 is 5000, the optimal mass ratio of prescr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com