Neutral complex nanoparticles composed of poloxamer and/or poloxamine and lipid
A technology of poloxamine and poloxamer, which is applied in the field of neutral complex nanoparticles, in vivo and in vitro cell gene transfection, and can solve the problems of high toxicity in vivo, unfavorable targeted modification, inactivation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1: Preparation of neutral complex nanoparticles of poloxamer and / or poloxamine combined with lipids
[0055] Recipe 1: T304:17R4:PC molar ratio is 172:105:1
[0056] First, take T304 and 17R4 out of the refrigerator at 4°C and equilibrate to room temperature, weigh them at room temperature according to the molar ratio of 172:105, add nuclease-free ultrapure water to dissolve, fully shake for 5 minutes with a mediator, and obtain stock solution A; Take PC out of the refrigerator at -20°C, equilibrate to room temperature, unseal, weigh at room temperature, dissolve with ethanol at 40°C, add nuclease-free ultrapure water drop by drop, ultrasonicate for 60 minutes, and transfer to a dialysis bag with MWCO of 10,000 Dialysis was performed with nuclease-free ultrapure water for 24 hours, and the dialysate was changed every 6 hours. After dialysis, filter with a 0.22um water-based filter membrane to obtain stock solution B. Mix stock solution A and stock solution B at...
Embodiment 2
[0069] Embodiment two: the characterization of prescription
[0070] The particle size and potential of the neutral complex nanometer involved in the present invention are tested by Malvern Zetasizer Nano ZSE, and the neutrality of prescription 1, prescription 2, prescription 3, prescription 4, prescription 5, prescription 6 and prescription 7 without FLuc-mRNA The complex nanoparticles were made into 1ml of the test solution, and the neutral complex nanoparticles without FLuc-mRNA were investigated under the condition of 25°C for prescriptions 1, 2, 3, 4, 5, 6 and 7. The particle size (Intensity Mean), surface potential (Zeta Potential) and polydispersity (PDI) of the dynamic light scattering nanoparticles.
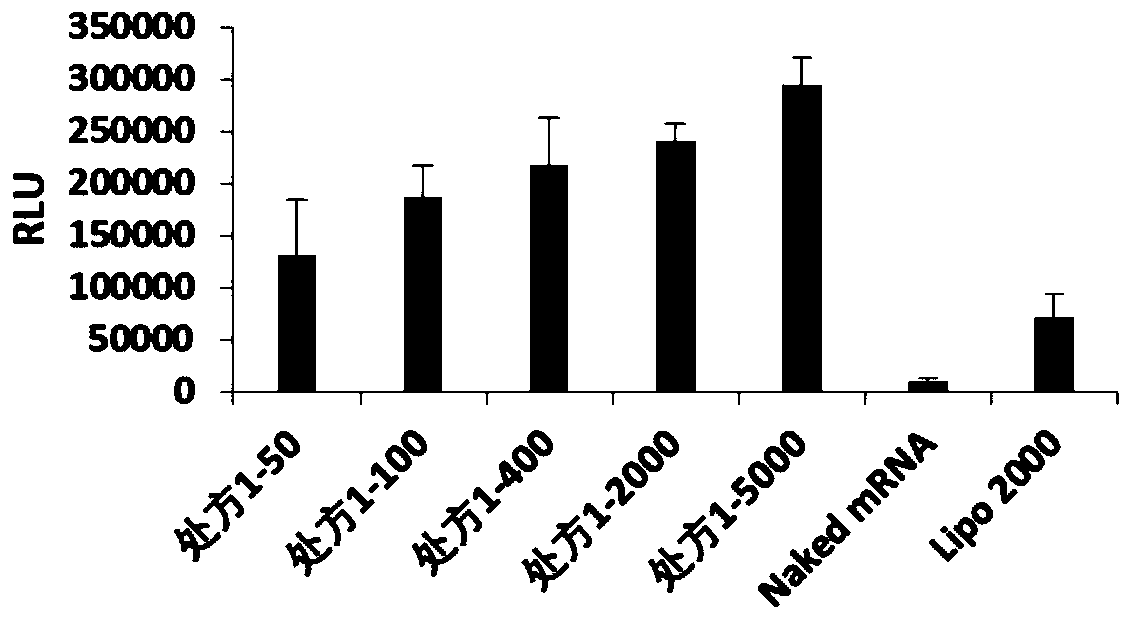
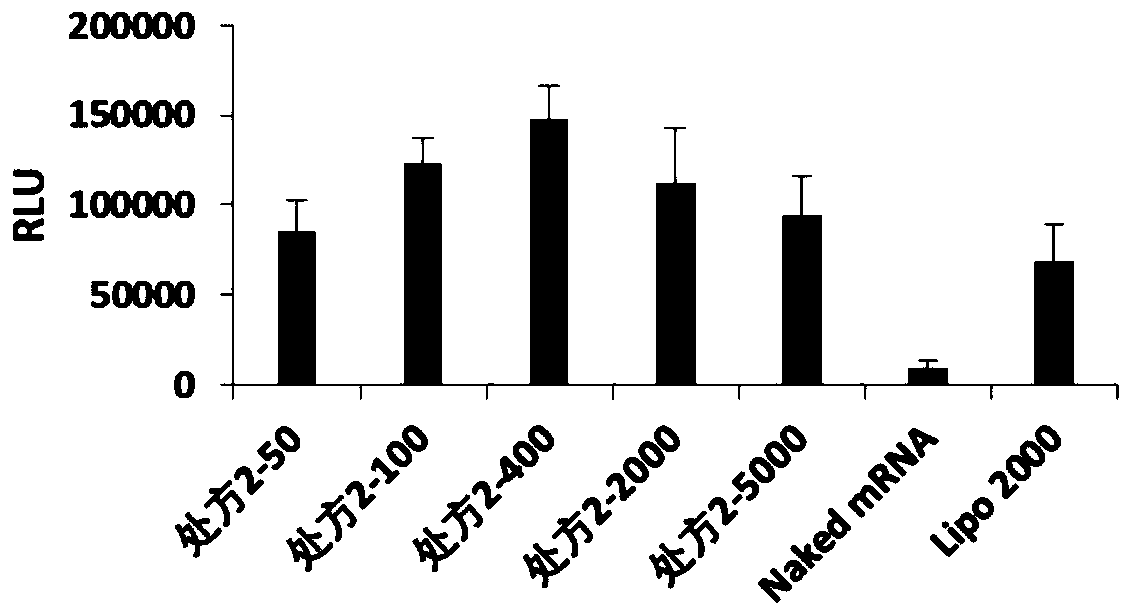
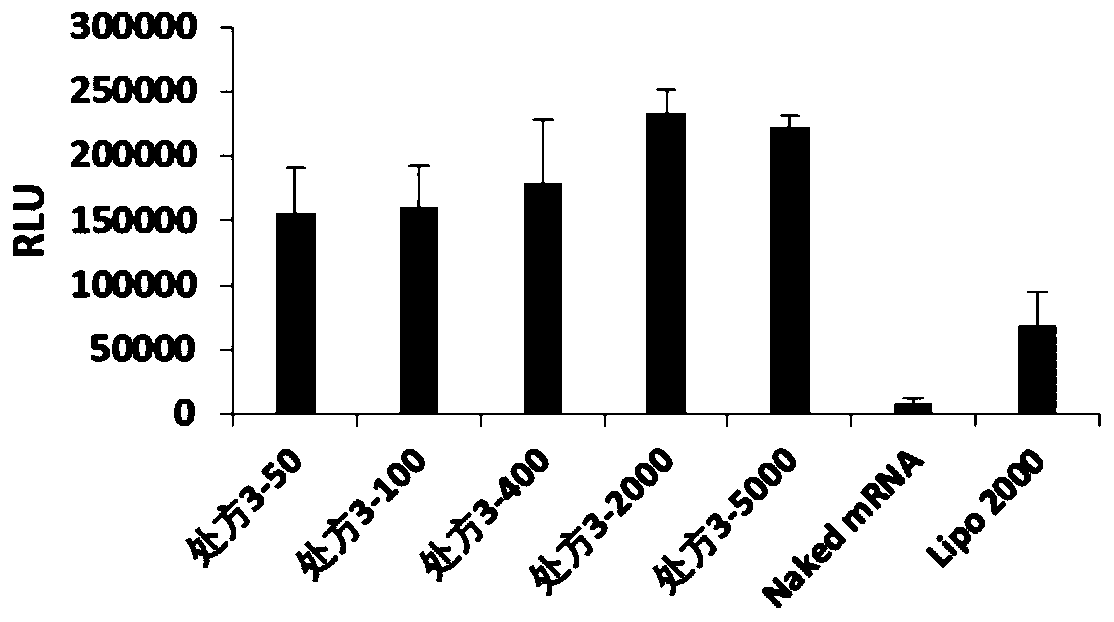
[0071] According to the optimal mass ratio of nanoparticles and FLuc-mRNA in each prescription (the optimal mass ratio of prescription 1 is 5000, the optimal mass ratio of prescription 2 is 400, the optimal mass ratio of prescription 3 is 2000, the optimal mass ratio of ...
Embodiment 3
[0076] Example 3: Encapsulation efficiency of various formulations of neutral complex nanoparticles of poloxamer and / or poloxamine combined with lipids
[0077] Quant-iT RiboGreen RNA Detection Kit (ThermoFische Company) was used to measure the encapsulation rate of FLuc-mRNA in each prescription (prescription 1, prescription 2, prescription 3, prescription 4, prescription 5, prescription 6 and prescription 7). For specific methods, refer to Kit instructions, the brief processing method of the present invention is:
[0078] According to the optimal mass ratio of nanoparticles and FLuc-mRNA in each prescription (the optimal mass ratio of prescription 1 is 5000, the optimal mass ratio of prescription 2 is 400, the optimal mass ratio of prescription 3 is 2000, the optimal mass ratio of prescription 4 is 5000, The optimal mass ratio of prescription 5 is 2000, the optimal mass ratio of prescription 6 is 100 and the optimal mass ratio of prescription 7 is 400) Weigh the neutral comp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com