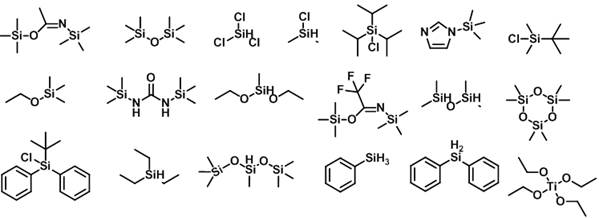
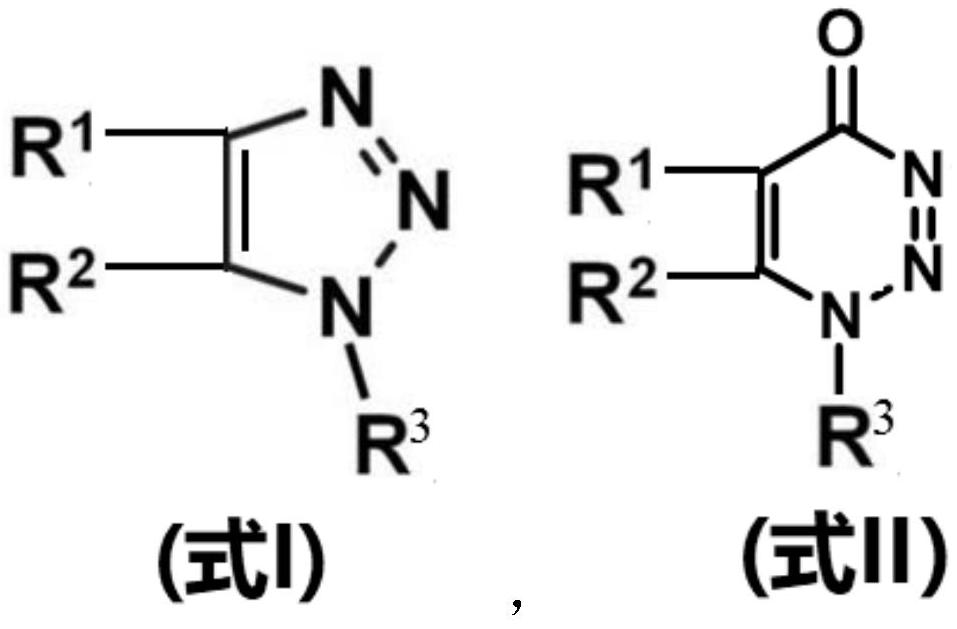
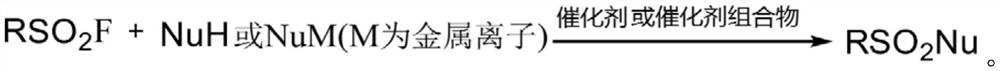Catalyst composition and use of catalyst composition or catalyst for catalyzing nucleophilic substitution reactions
A technology of catalysts and compositions, applied in catalytic reactions, physical/chemical process catalysts, compounds of Group 5/15 elements of the periodic table, etc., which can solve the problems of non-compliance with green chemistry, increased production costs, limited substrate range, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073]
[0074] Add 3-methyl-6-(diphenylphosphine)benzenesulfonyl fluoride (0.2mmol, 1.0eq), DMSO (250μL), tert-butylamine (63.0μL, 3.0eq), HOBt (27.0mg, 1.0 eq). The reaction mixture was heated to 40°C and stirred for 24 hours. After the reaction stopped. The yield calculated by nuclear magnetic phosphorus spectrum was 87%.
Embodiment 2
[0076] Except that the catalyst HOBt was replaced by PyBOP, the reaction was carried out in the same manner as in Example 1, and the yield calculated by nuclear magnetic phosphorus spectrum was 93%.
Embodiment 3
[0078] Except that the catalyst HOBt was replaced by HOCT, the reaction was carried out in the same manner as in Example 1, and the yield calculated by nuclear magnetic phosphorus spectrum was 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com