Pharmaceutical preparation for use in treating epstein-barr virus positive patients with reactivation phenomenon-associated diseases
A technology of reactivation and disease, applied in antiviral agents, cell culture active agents, diseases, etc., can solve problems such as weak control, no disease treatment drugs, and unbalanced EBV immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
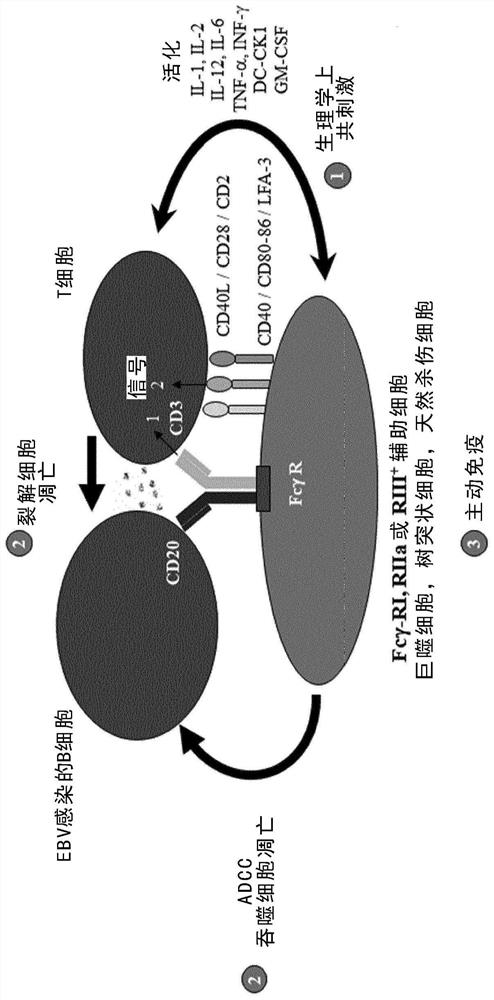
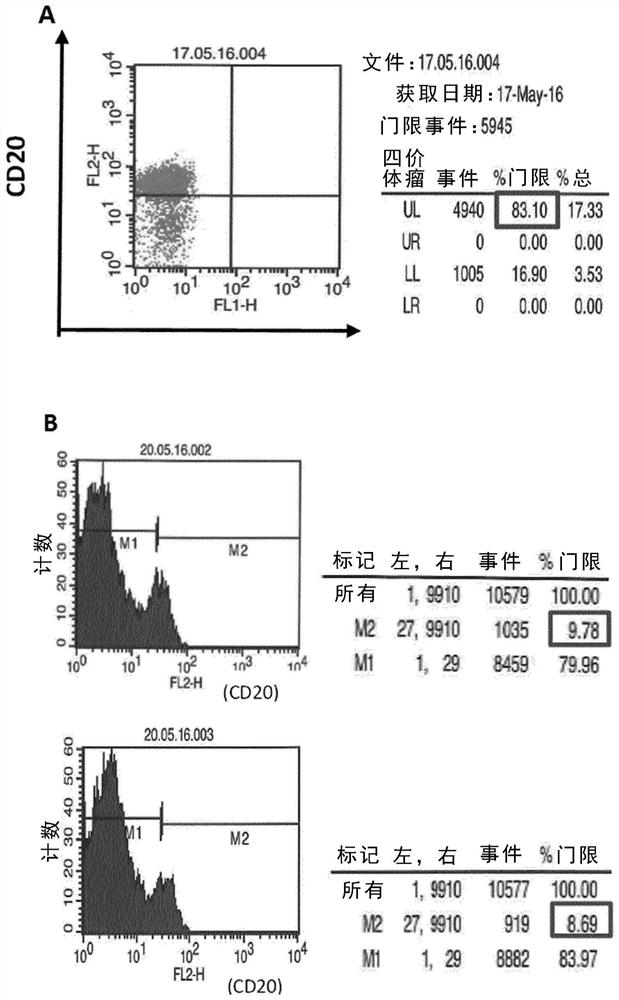
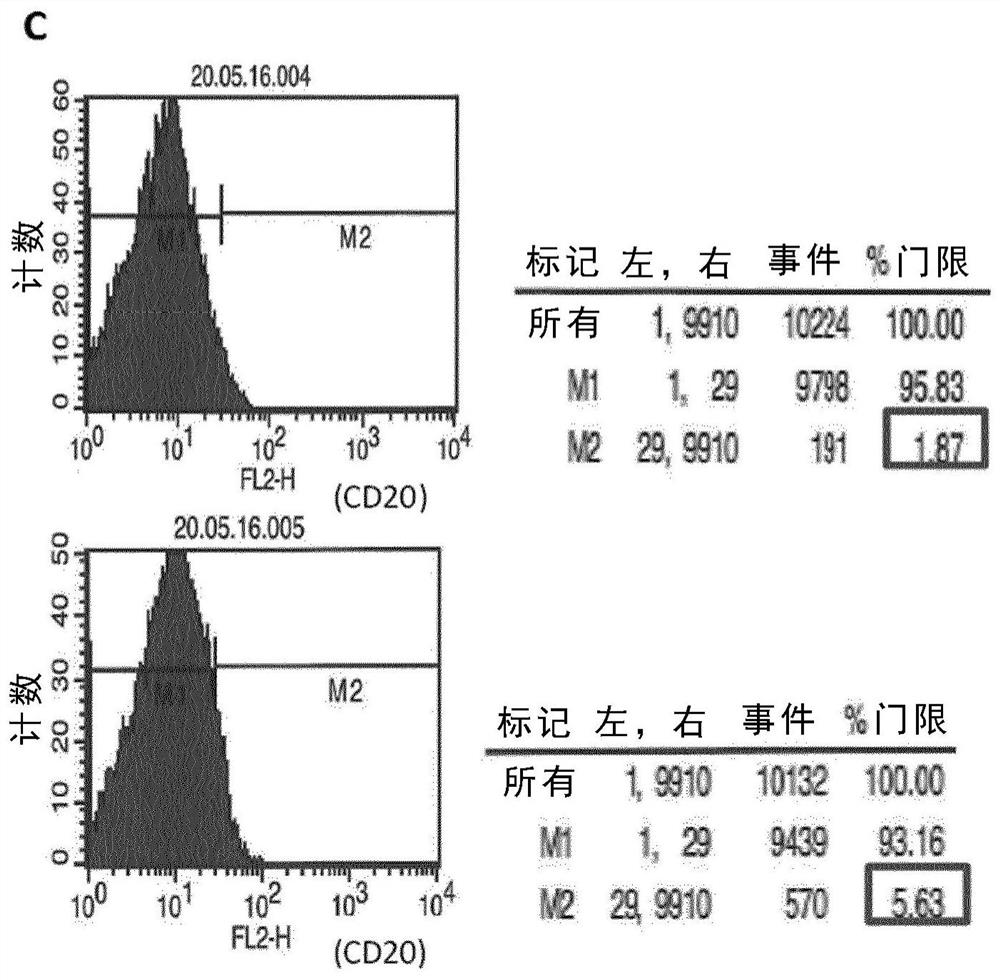
[0151] Example 1. In vitro killing of enriched autologous B cells mediated by trifunctional bispecific antibodies
[0152] Example 1 demonstrates efficient killing of enriched autologous B cells targeted in vitro by a CD20-specific trifunctional bispecific antibody. 250,000 enriched B cells and 500,000 PBMCs from healthy donors were prepared as described in the Methods and Materials section. Cells were mixed and incubated in the presence or absence of 1 μg Bi20 as a trifunctional bispecific anti-CD3x anti-CD20 antibody ( figure 1 ). Incubate the cells at 37 °C and 5% CO 2 The culture medium (RPMI1640 medium supplemented with 8.9% FCS, 2 mM L-glutamine, 1 mM sodium pyruvate and 1× non-essential amino acids) was cultured in a total volume of 1 ml in a 24-well plate. Three days later, cells were analyzed by flow cytometry using FACS-Calibur and Cellquest pro software (Becton Dickinson, USA). B cells were stained by PE (phycoerythrin)-conjugated anti-human CD20 monoclonal anti...
Embodiment 2
[0153] Embodiment 2 (chronic fatigue syndrome, chronic dry cough, night sweats)
[0154] A 49-year-old woman who tested positive for EBV suffered from repeated episodes of fatigue for ten years, lymphedema, chronic otitis media, and frequent nocturnal sweating. In addition, the patient was diagnosed with pancreatitis in May 2010 and developed rectal cancer in July 2010. EBV was detected in tumor cells and subsequently in surgical scars by PCR analysis. Since 2000, the patient suffered from chronic dry cough.
[0155] In a detailed EBV diagnosis, for example, a high frequency of EBER-1+2 CISH positive B cells (>50%) together with slightly elevated IL-8 values can be determined within the patient's enriched B cell fraction.
[0156] Because of this diagnosis and unmet medical need, the patient decided to enroll in compassionate use treatment with an investigational anti-EBV therapeutic vaccine.
[0157] For the preparation of vaccine cell preparations, 60 ml of peripheral b...
Embodiment 3
[0176] Example 3 (Insulin Resistance, Type 2 Diabetes, Potency Issues)
[0177] A 50-year-old man who tested positive for EBV and herpesvirus types 1 and 2, CMV, and chlamydial pneumonia had hyperinsulin resistance, elevated liver enzymes, abnormal liver metabolic function, hypercholesterolemia, hypertriglycerides, Metabolic syndrome of sleep disturbance, fatigue, poor concentration and potency problems.
[0178] In detailed EBV diagnosis, for example, a high frequency of EBNA-1+2 CISH positive B cells (50-75%) can be detected within the enriched B cell fraction of a patient. Because of this diagnosis and an unmet medical need, the patient decided to participate in compassionate use treatment with an investigational anti-EBV therapeutic vaccine, also as described in Example 2.
[0179] Cell preparations similar to Examples 1 and 2 were generated ex vivo and administered back to the patient subcutaneously (May 13, 2015).
[0180] For enhanced application, the same procedure w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com