Preparation method of tamsulosin hydrochloride crystal form
A technology of tamsulosin crystal form and hydrochloric acid, which is applied in the preparation of sulfonamides, organic chemistry methods, organic chemistry, etc., can solve problems affecting clinical efficacy and drug quality, affecting dissolution speed and stability, and different solubility , to achieve the effect of high product purity, high yield and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] The embodiment of the present invention provides a preparation method of tamsulosin hydrochloride crystal form, comprising:
[0026] Step S11, adding tamsulosin to the solvent, raising the temperature to the first temperature, and obtaining the first solution after being completely dissolved;
[0027] Step S12, adding an acid to the first solution, and obtaining a second solution after keeping warm at the first temperature;
[0028] Step S13, cooling the second solution to a second temperature, stirring and reacting to obtain a third solution;
[0029] Step S14, after filtering and drying the third solution, the crystal form of tamsulosin hydrochloride is obtained.
[0030] Further, in step S11, the solvent includes at least one of acetonitrile, ethanol, acetone and tetrahydrofuran, preferably the solvent is acetonitrile.
[0031] The first temperature is 30°C to 85°C, for example, 30°C, 31°C, 32°C, 33°C, 34°C, 35°C, 40°C, 45°C, 50°C, 60°C, 70°C, 80°C, 85°C, etc., pr...
Embodiment 1
[0041] Step S1: Add 5g of tamsulosin into the reaction flask, then add 50mL of acetonitrile, heat up to 60-70°C, and completely dissolve to obtain the first reaction solution;
[0042] Step S2: Add 7.5 g of concentrated hydrochloric acid dropwise to the above first reaction solution, and keep the reaction for 1 hour under the condition of 60-70° C. to obtain the second reaction solution;
[0043] Step S3: Slowly cool the second reaction solution to about 25°C, and then stir for 5 hours to obtain a third reaction solution;
[0044] Step S4: Filter and dry the above-mentioned third reaction solution to obtain 5.17 g of a white solid, which is the crystal form of tamsulosin hydrochloride, HPLC: 99.98%, and the yield is 95%.
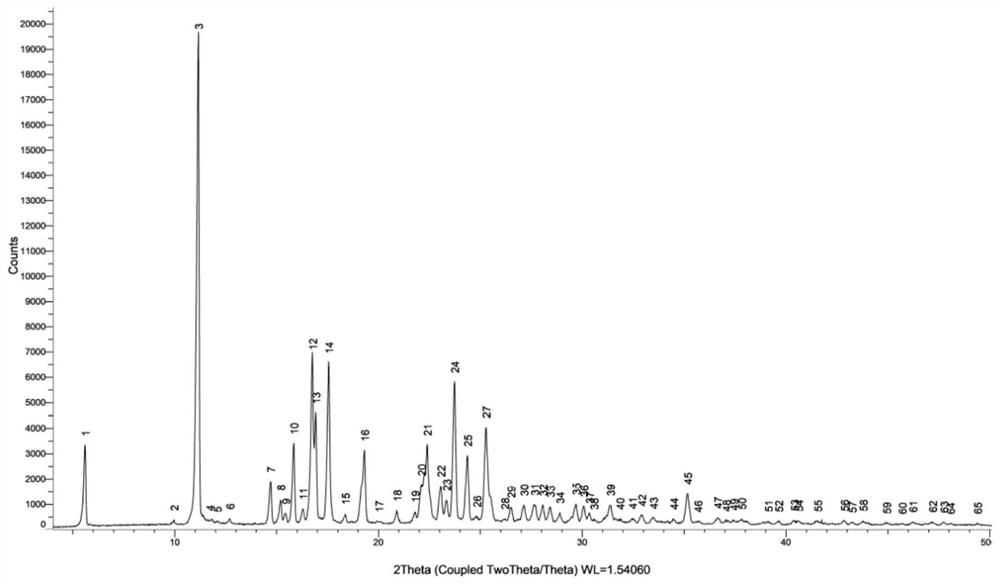
[0045] The XRD pattern of tamsulosin hydrochloride crystal form is shown in figure 1 , the testing instrument is D2 Phaser X-ray powder diffractometer. The test conditions: anode target, Cu target; tube pressure 30kV, tube current 10mA; 2θ scanning range 4...
Embodiment 2
[0048] Step S1: Add 5g of tamsulosin into the reaction flask, then add 50mL of ethanol, heat up to 70-75°C, and completely dissolve to obtain the first reaction solution;
[0049] Step S2: Add 7.5 g of concentrated hydrochloric acid dropwise to the above first reaction solution, and keep warm for 1 hour under the condition of 70-75° C. to obtain the second reaction solution;
[0050] Step S3: Slowly cool the second reaction solution to about 25°C, and then stir for 5 hours to obtain a third reaction solution;
[0051] Step S4: Filter and dry the above third reaction solution to obtain 4.95 g of white solid, which is the crystal form of tamsulosin hydrochloride, HPLC: 99.89%, yield 91%.
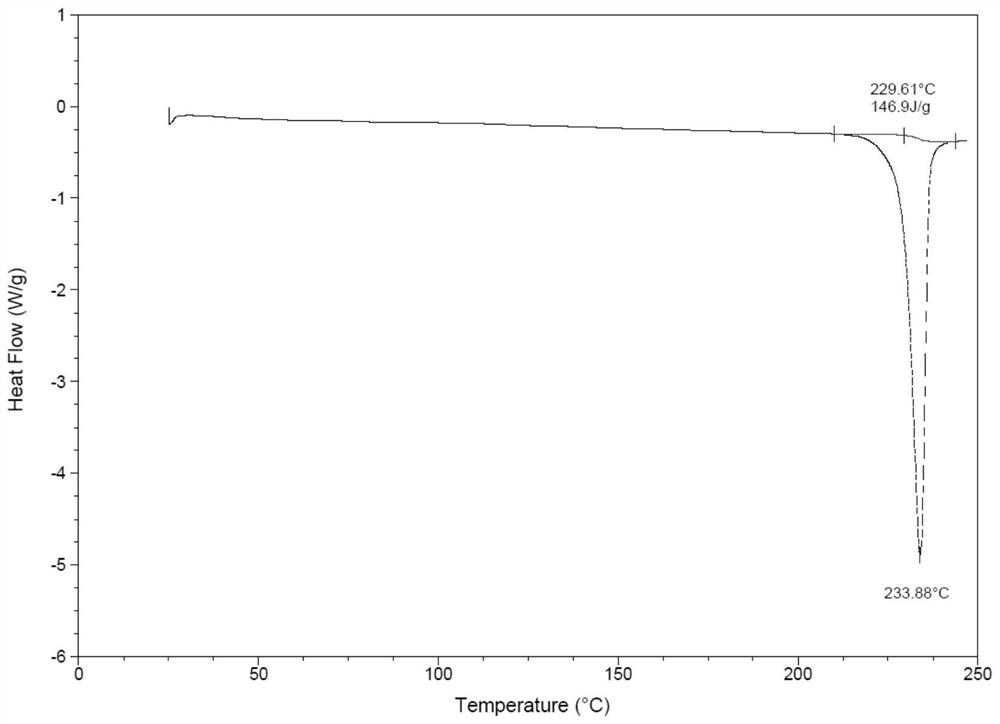
[0052] For the XRD pattern and DSC pattern of tamsulosin hydrochloride crystal form, refer to Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com