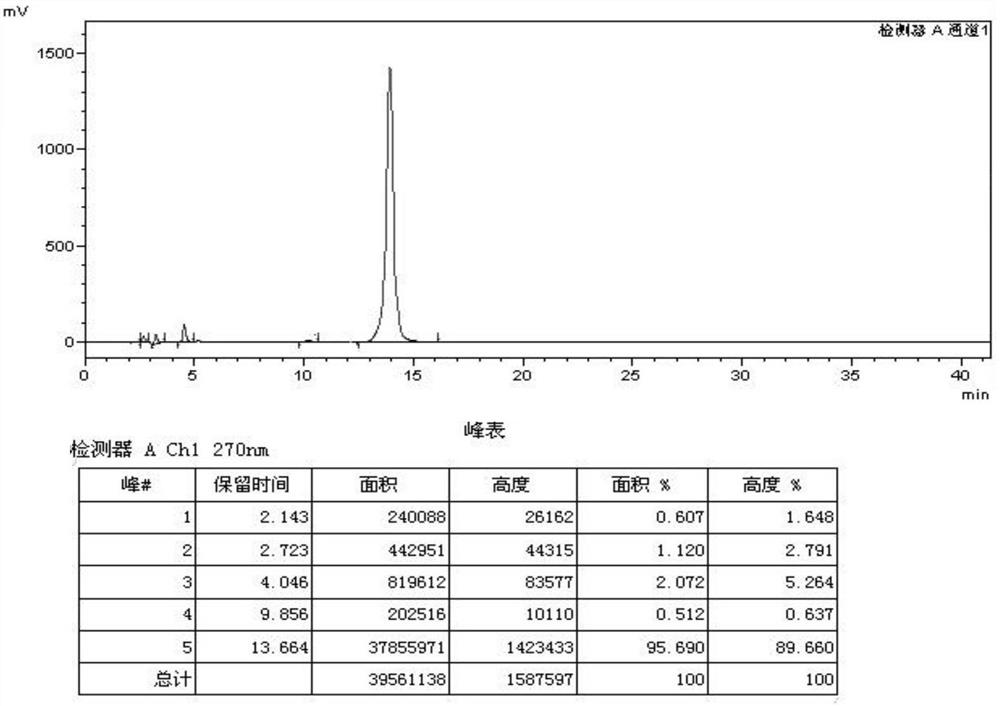
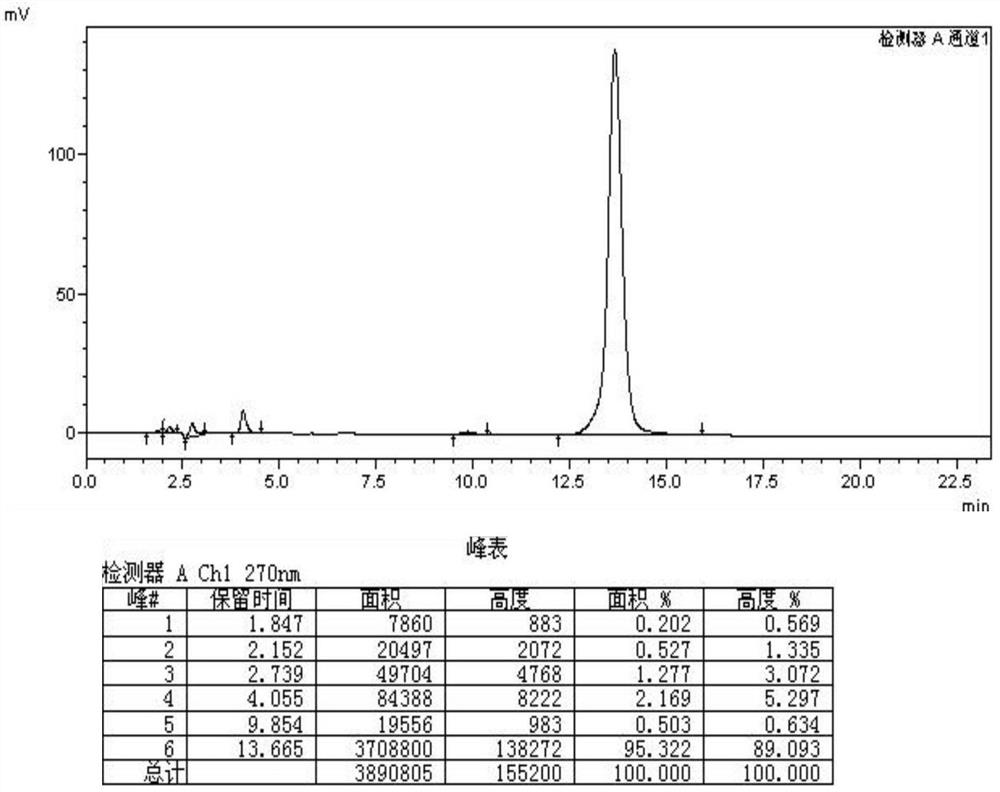
Preparation method for producing cefquinome sulfate injection through combination of emulsification stirring tank and colloid mill
A technology of cefquinome sulfate and colloid mill, which can be applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and devices for making drugs into special physical or taking forms, which can solve the problem of drug preparation time long-term, poor drug quality stability, low yield, etc., to achieve the effect of fine particle size, ensure quality stability, and improve yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] A kind of preparation method that utilizes emulsification stirring tank and colloid mill to produce cefquinome sulfate injection, comprises the following steps: form according to the mode of following weight ratio: cefquinome sulfate 2.5% (g / ml); Stearin Aluminate 2.0% (g / v); Lecithin 1.0% (g / v); Vitamin E 0.2% (g / v); Injection grade ethyl oleate plus total volume.
[0043] 1. Add aluminum stearate to injection-grade ethyl oleate (80% v / v), and stir evenly in the emulsification mixing tank, heat the steam interlayer to 121°C, and keep stirring. After constant temperature for 30 minutes, use Cool the drinking water interlayer to room temperature, turn on the emulsifier on the emulsification tank, and emulsify for 30 minutes.
[0044] 2. Add cefquinome sulfate, lecithin, and vitamin E to (1), and turn on the emulsifier in the emulsification tank to emulsify for 30 minutes.
[0045] 3. Emulsify the emulsified medicinal solution in a colloid mill, grind it for 30 minutes, ...
Embodiment example 2
[0055] Form according to the following weight ratio: cefquinome sulfate 5% (g / ml); aluminum stearate 3% (g / v); lecithin 2% (g / v); vitamin E 0.5% ( g / v); injection grade ethyl oleate plus total volume.
[0056] 1. Add aluminum stearate to injection-grade ethyl oleate (80% v / v), and stir evenly in the emulsification mixing tank, heat the steam interlayer to 121°C, and keep stirring. After constant temperature for 30 minutes, use Cool the drinking water interlayer to room temperature, turn on the emulsifier on the emulsification tank, and emulsify for 30 minutes.
[0057] 2. Add cefquinome sulfate, lecithin, and vitamin E to (1), and turn on the emulsifier in the emulsification tank to emulsify for 30 minutes.
[0058] 3. Emulsify the emulsified medicinal solution in a colloid mill, grind it for 30 minutes, and then let it rest for 1 hour, then dissolve it with ethyl oleate to a sufficient amount, and recirculate it for emulsification and grinding for 30 minutes.
[0059] 4. Af...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com