Application of thrombospondin 1 (THBS-1) in preparation of medicine for preventing and/or treating age-related macular degeneration (AMD)
A reactive protein, macular degeneration technology, applied in the direction of drug combination, peptide/protein composition, pharmaceutical formulation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
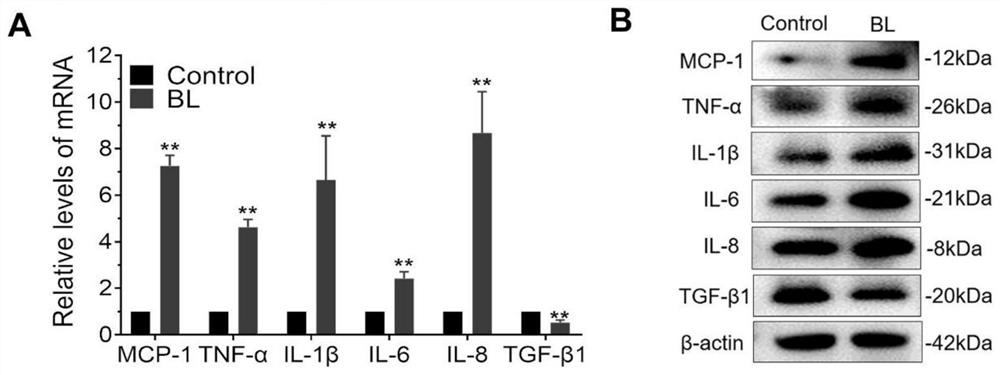
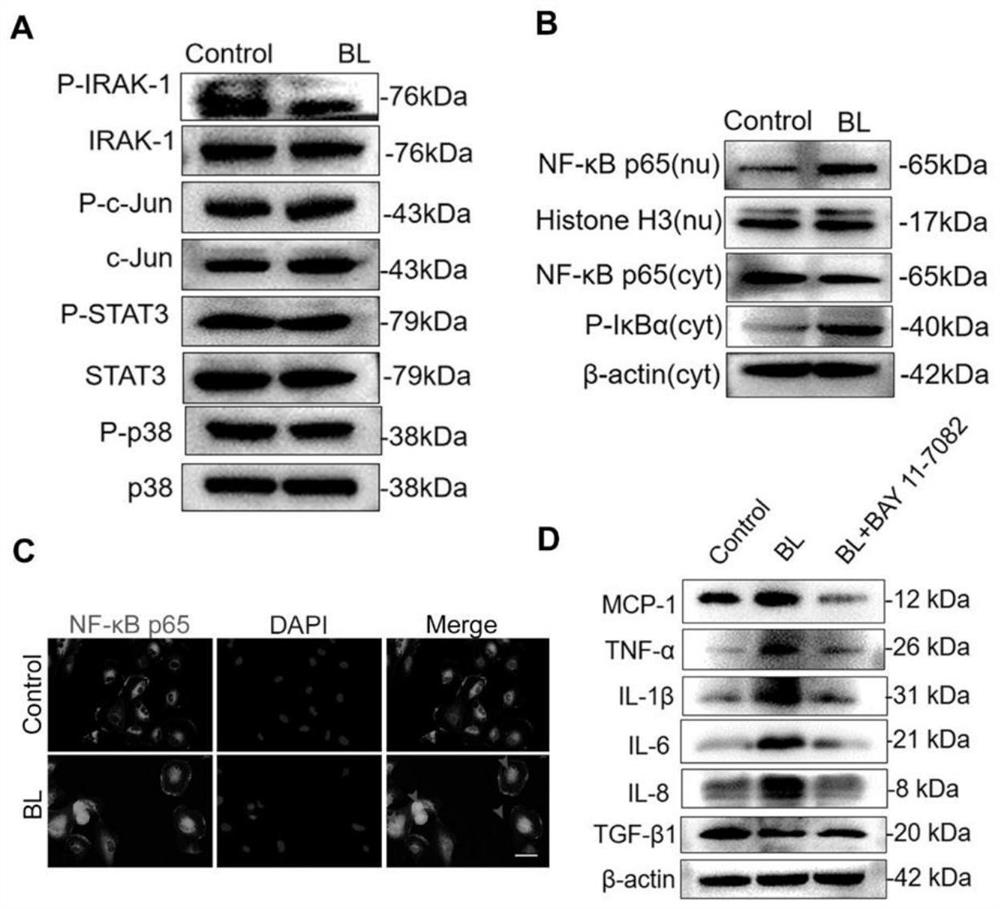
[0038] Thrombospondin 1 (THBS-1) can effectively inhibit the retinal inflammatory response induced by blue light
[0039] 1. Cell culture
[0040] ARPE-19 cells (human retinal pigment epithelial cell line) were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences. Cells were cultured in high-glucose DMEM medium containing 10% FBS and 1% penicillin-streptomycin double antibody at 37°C and 5% CO 2 Cultured in an incubator, and after about 2 to 3 days, the cells were subcultured at a ratio of 1:3.
[0041] 2. THBS-1 administration treatment
[0042] For in vitro experiments, 4 nM recombinant human thrombospondin-1 (THBS-1) was added to cells in a serum-starved state and incubated for at least 6 hours before blue light irradiation.
[0043] 3. Description of cell grouping
[0044] THBS-1 administration treatment + blue light irradiation group: 8×10 3 The density of cells / well was seeded in a 96-well plate, and after the cells were treated according to the ...
Embodiment 2
[0076] Thrombospondin 1 (THBS-1) can effectively inhibit pathological neovascularization
[0077] 1. hMEC-1 cell level experiment
[0078] 1. Cell Culture
[0079] hMEC-1 cells (human microvascular endothelial cell line) were purchased from ATCC cell bank in the United States. Cells were cultured in ECM (endothelial cell medium) containing 5% FBS, 1% penicillin-streptomycin double antibody and 1% endothelial cell growth supplement (ECGS) at 37°C, 5% CO 2 Culture in an incubator, change the medium halfway every day, and perform cell passage at 1:2 after about 2 to 3 days.
[0080] 2. THBS-1 administration treatment
[0081] When detecting the expression of phosphorylated protein in hMEC-1 cells, the cells were pretreated with 4 μM THBS-1 solution for 6 h under serum starvation state, and 20 ng / mL VEGF was added to the medium without ECGS for 30 min.
[0082] 3. Cell grouping
[0083] THBS-1 administration treatment+VEGF treatment group: at a density of 8×10 3 The cells / we...
Embodiment 3
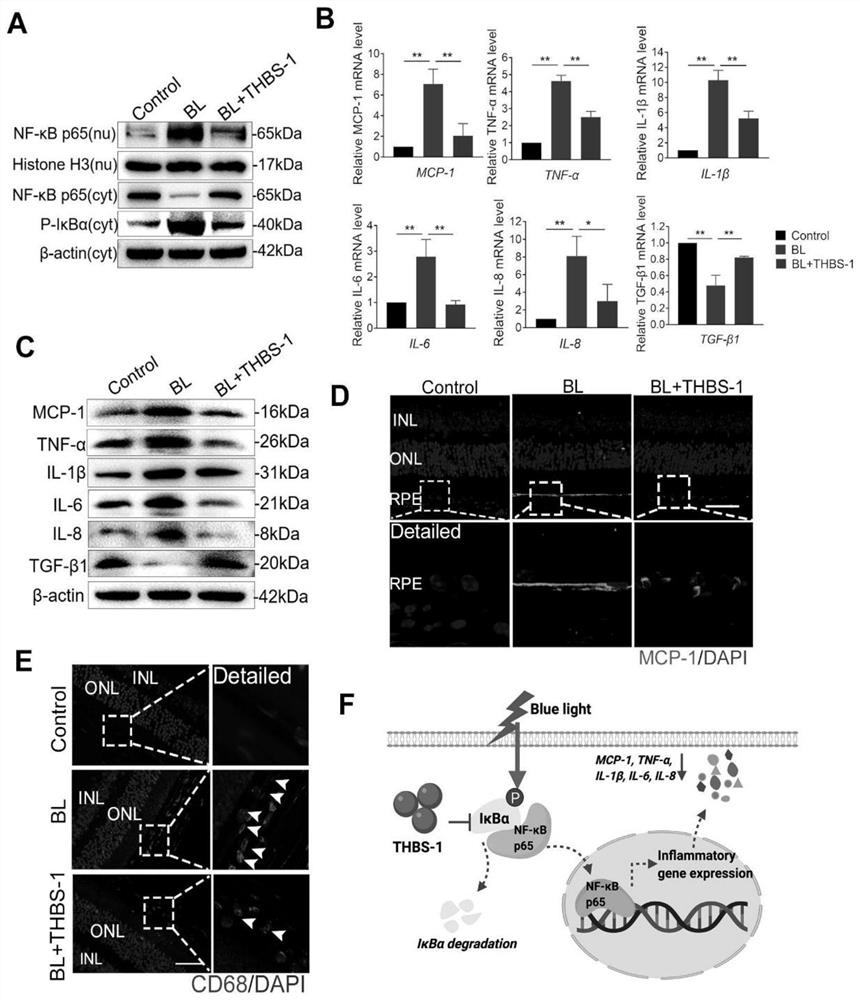
[0118] Thrombospondin 1 (THBS-1) significantly protects the retina from blue light-induced retinal damage
[0119] 1. ARPE-19 cell level experiment
[0120] 1. Cell culture
[0121] ARPE-19 cells (human retinal pigment epithelial cell line) were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences. Cells were cultured in high-glucose DMEM medium containing 10% FBS and 1% penicillin-streptomycin double antibody at 37°C and 5% CO 2 Cultured in an incubator, and after about 2 to 3 days, the cells were subcultured at a ratio of 1:3.
[0122] 2. THBS-1 administration treatment
[0123] For in vitro experiments, 4 nM recombinant human thrombospondin-1 (THBS-1) was added to cells in a serum-starved state and incubated for at least 6 hours before blue light irradiation.
[0124] 3. Cell grouping
[0125] THBS-1 administration treatment + blue light irradiation group: 8×10 3 The density of cells / well was seeded in a 96-well plate, and after the cells were trea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com