Racemization recovery method of dextromethorphan hydrobromide intermediate byproducts
A technology of dextromethorphan hydrobromide and recovery method, which is applied in the direction of organic chemistry, can solve the problems of cost increase, environmental pollution, raw material loss, etc., and achieve the effects of reducing production cost, simplifying the process flow, and shortening the production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
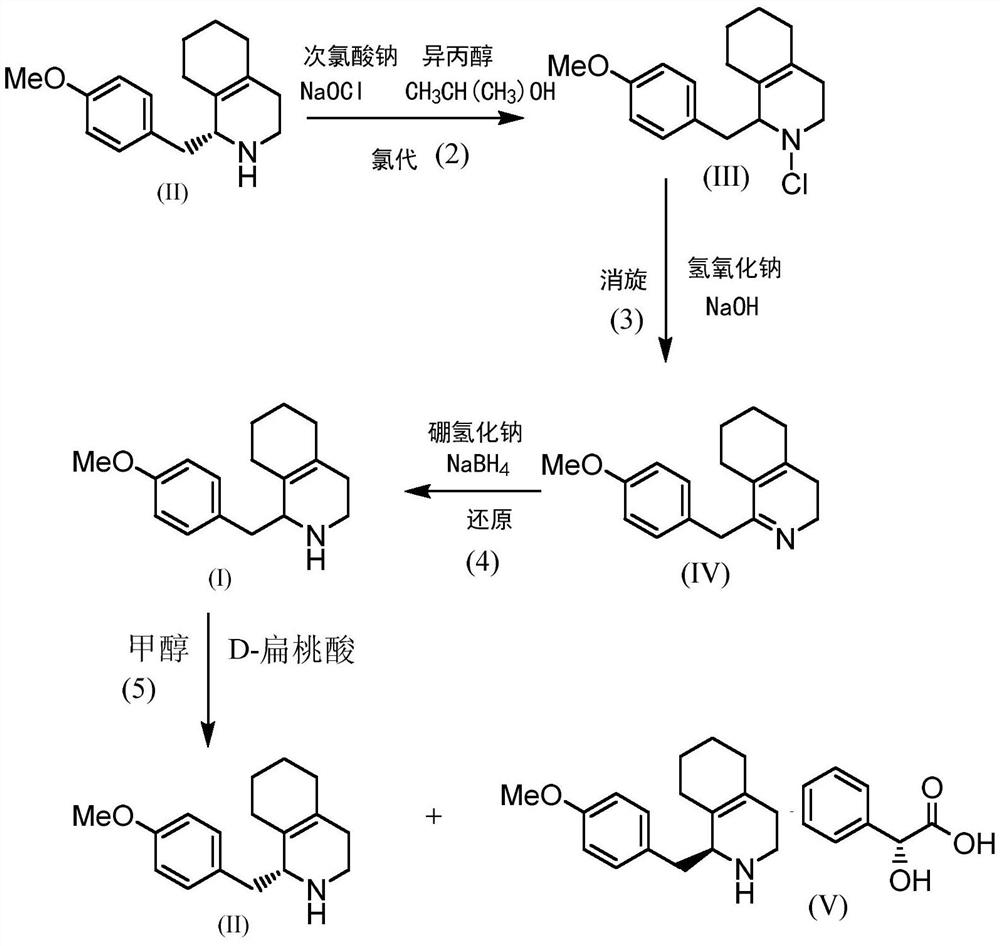
[0019] A kind of racemization recovery method of dextromethorphan hydrobromide intermediate by-product, specifically comprises the following steps
[0020] 1) Mother liquor treatment: the mother liquor to be treated contains (S)-1,2,3,4,5,6,7,8-octahydro-1-[(4-methoxyphenyl)methyl]isoquinoline The crystallization mother liquor and washing liquid of phenoline (II), there is 45Kg compound (II) in the mother liquor to be treated, and the organic solvents in the mother liquor to be treated are methanol and toluene; the treatment mode of the mother liquor to be treated is: under stirring conditions, controlled at the liquid temperature 40°C, distilled under reduced pressure until the methanol is basically evaporated, keeping the liquid temperature at 40°C; when the liquid temperature of the concentrated mother liquor is less than 40°C, add sodium hydroxide solution, stir for 5min, let stand for 1h, check that the pH is greater than 12, and then separate layers, The water phase was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com