Intermediate for synthesizing 2-chloroadenosine, synthesis process of intermediate and synthesis process of 2-chloroadenosine
A synthesis process, chloradenosine technology, applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of large pollution, harsh reaction conditions, high price, etc., avoid heavy metal catalysts, and be conducive to industrialization The effect of easy production and reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
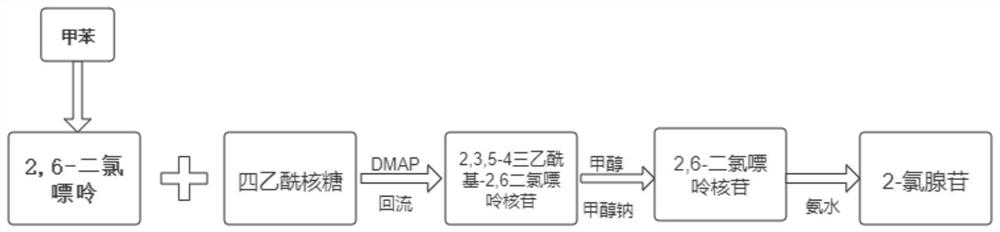
[0052] see figure 1 , the present embodiment provides a synthesis process of 2-chloroadenosine, comprising the following steps:
[0053] S1, condensation reaction;
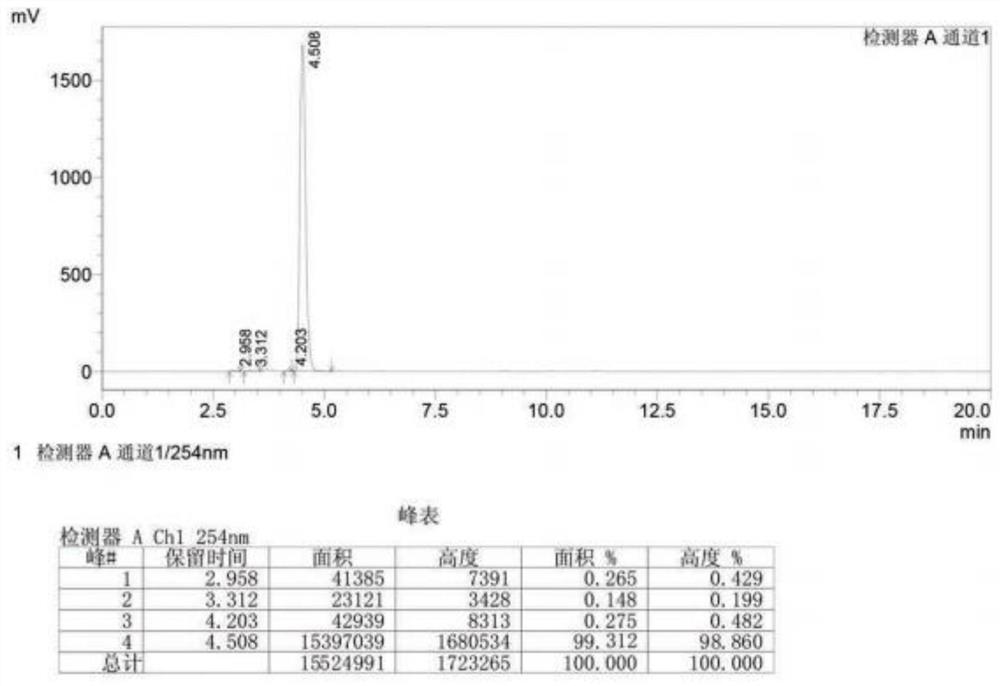
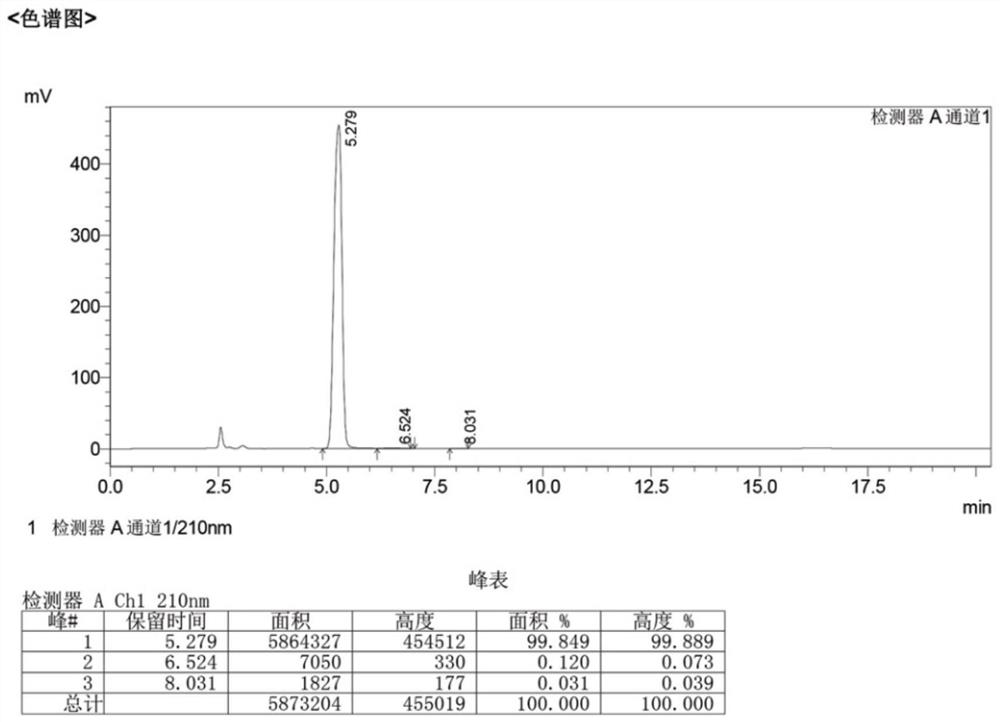
[0054] Take 189g of 2.6-dichloropurine, add it to 750ml of toluene, then add 318g of tetraacetyl ribose, 0.9g of dmap, heat up to 110°C and reflux for 2h, after the reaction, cool the reaction solution to room temperature for crystallization, and get 2,3 ,5-4 triacetyl-2,6 dichloropurine nucleoside, 436.6g, yield 97.5%, purity 99.8%. Perform high-fluid analysis on it, and the test results can be found in image 3 .
[0055] S2, hydrolysis reaction;
[0056] Add 2,3,5-4 triacetyl-2,6 dichloropurine nucleoside (426.6g) synthesized by S1 into 1700ml of methanol, then add 62g of sodium methoxide, and react at 25°C for 5h. After the reaction, add 1.5L of water, cooled to 0°C for crystallization to obtain 2.6-dichloropurine nucleoside, 300.0g.
[0057] S3, ammoniation reaction;
[0058] Add 2.6-dichloropurine nuc...
Embodiment 2-12
[0061] Example 2-12 Synthesize 2-chloroadenosine according to the synthesis process provided in Example 1, the difference is that the operating conditions are different, as shown in Table 1-Table 3 below:
[0062] Table 1 Changes in DMAP dosage
[0063]
[0064] Table 2 Proportion changes
[0065]
[0066] Table 3 Proportion and temperature change
[0067]
[0068]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com