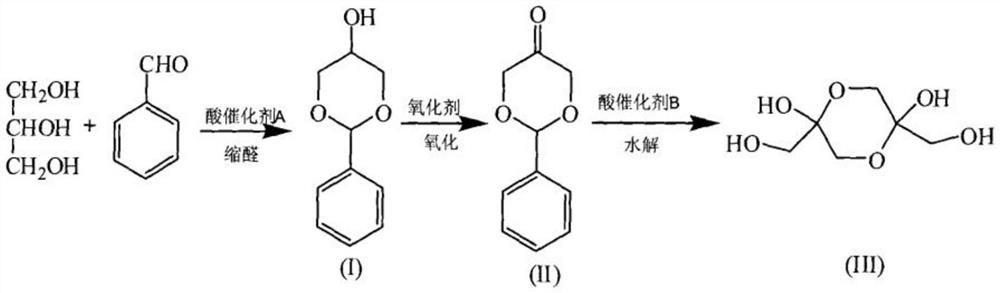
Preparation method of benzaldehyde glycerol acetal
A technology of benzaldehyde and glycerin, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, catalytic reactions, etc., to achieve high activity, high selectivity, and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
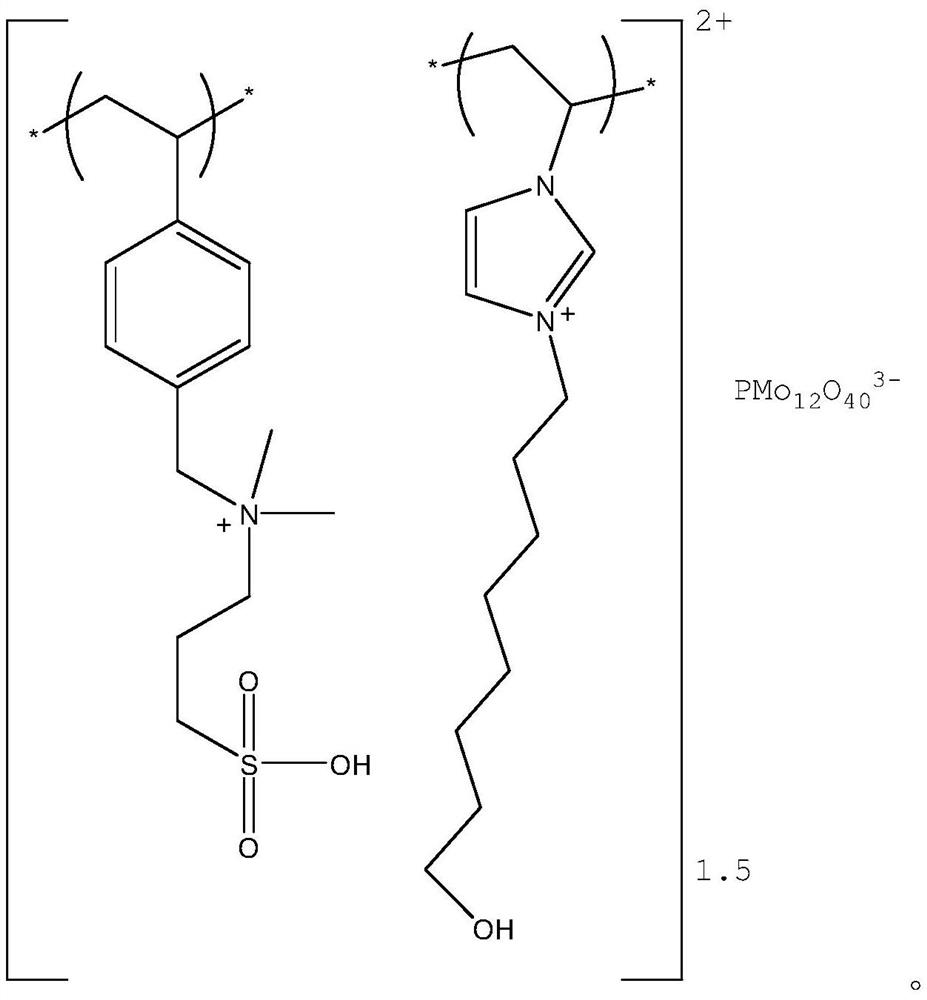
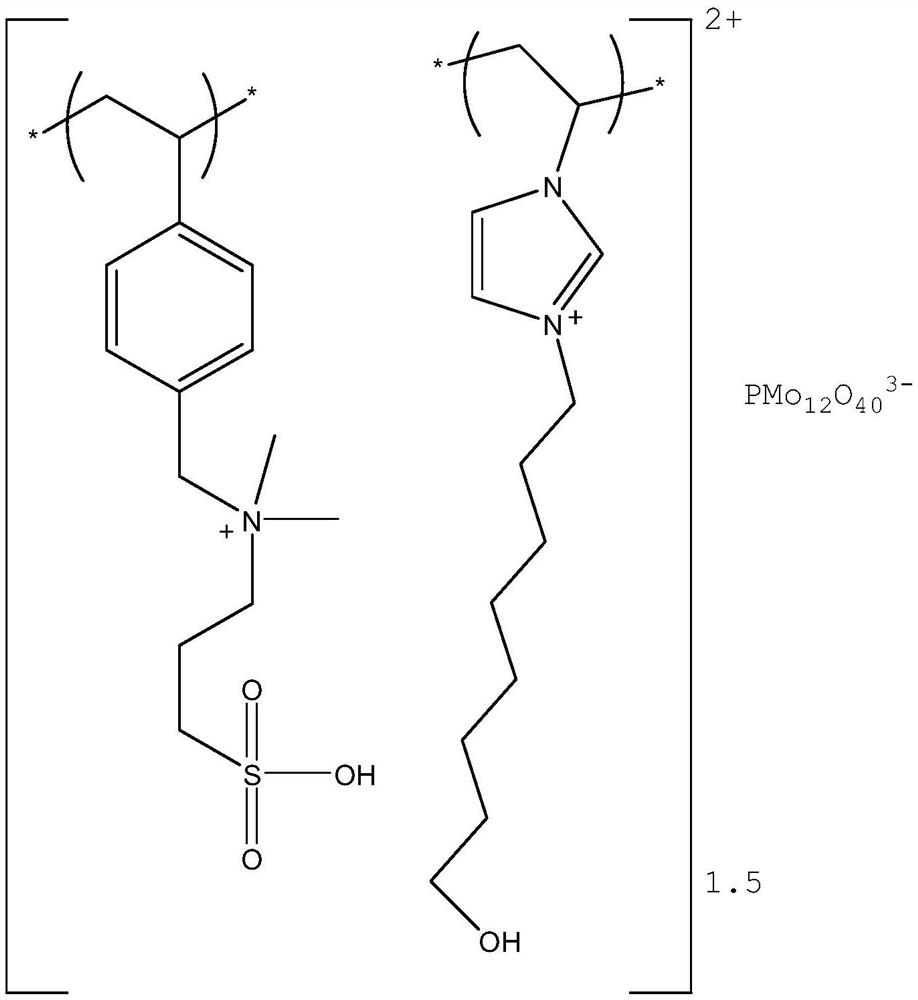
[0035] Preparation of Polymerized Ionic Liquid Catalyst:
[0036] (1) Preparation of sulfonic acid functional cationic monomer
[0037]
[0038] Under the protection of nitrogen, add N-4-vinylbenzyl-N,N-dimethylamine (9g) into a reaction kettle with 60ml of absolute ethanol, stir at room temperature, and continuously and slowly drop into 1,3-propanesulfonic acid Esters (6.2g), after the dropwise addition, the system was heated up to 80°C at a rate of 1°C / min, and reacted overnight; then cooled to room temperature, the solvent was removed under reduced pressure, washed with dichloromethane to remove unreacted raw materials, and dried to obtain light Brown viscous liquid product, yield 98%. 1 H NMR (400MHz, CDCl 3 )δ=2.21(m,2H,CH 2 ), 3.18 (m,2H,CH 2 ), 3.34 (s,6H,CH 3 ), 3.53(t,2H,CH 2 ), 4.30 (s,2H,CH 2 ), 5.12 (d,2H,=CH 2 ), 6.12 (t, H, =CH), 6.96 (d, 2H, CH), 7.19 (d, 2H, CH).
[0039] (2) Preparation of hydroxyl-functionalized imidazolium cationic monomer
[00...
Embodiment 2
[0049] With the H of step (4) in embodiment 1 3 PMo 12 o 40 Phosphomolybdic acid was replaced by an equivalent amount of H 3 PW 12 o 40 Phosphotungstic acid, other with embodiment 1.
Embodiment 3
[0051] The 8-chloro-n-octanol of step 2 in the embodiment is replaced by an equivalent 8-chloro-n-octane, and the others are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com