Method for synthesizing Etelcalcetide
A synthesis method and compound technology, applied in the field of peptide synthesis, can solve problems such as coupling difficulties and reduce production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
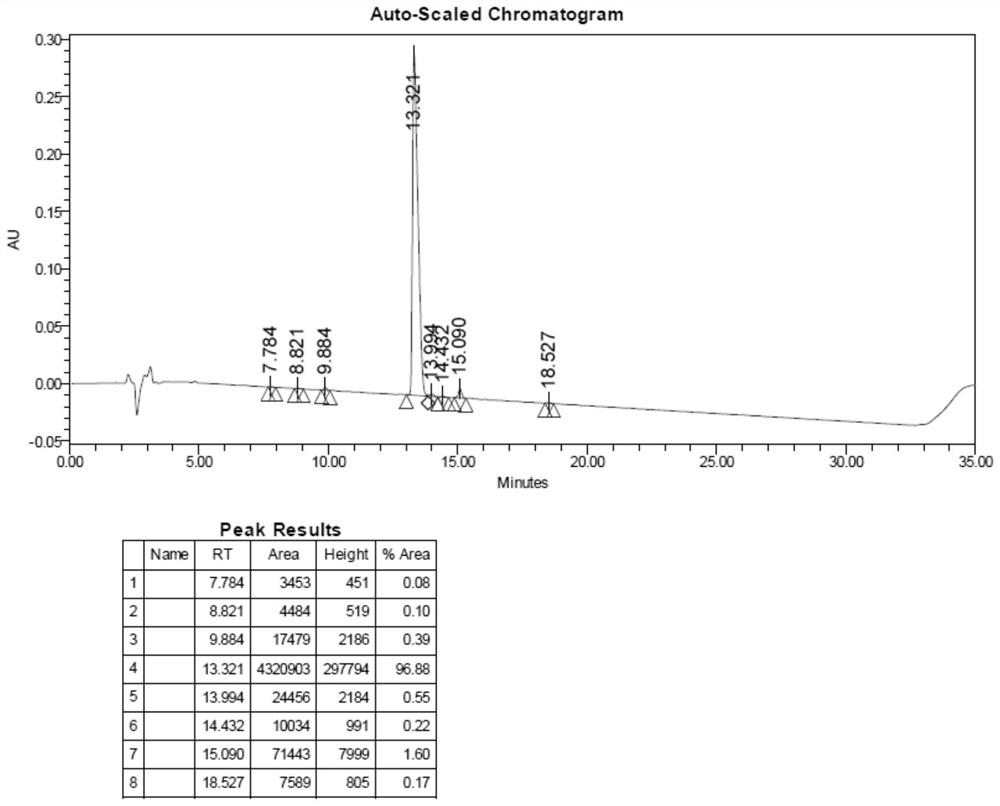
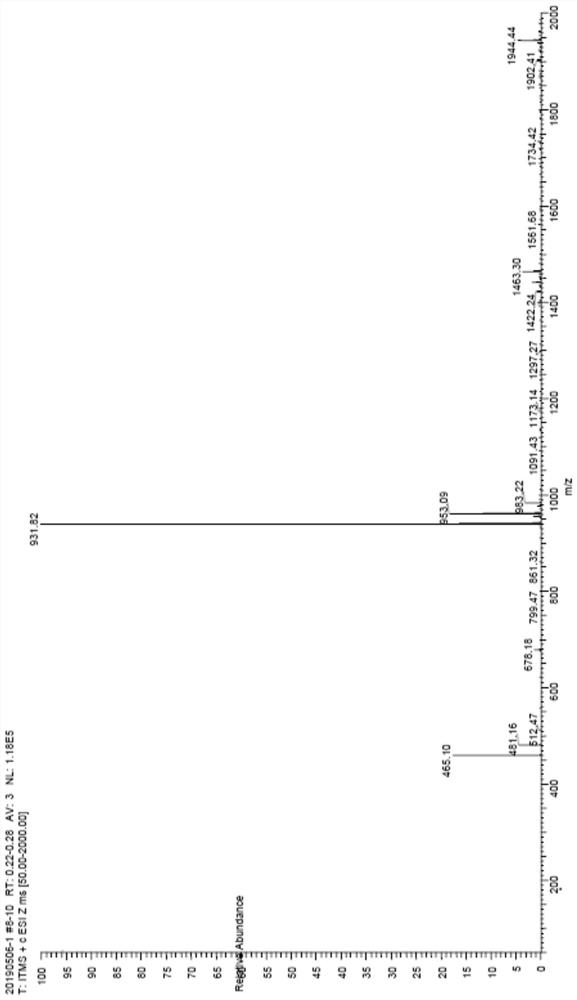
Examples
Embodiment 1
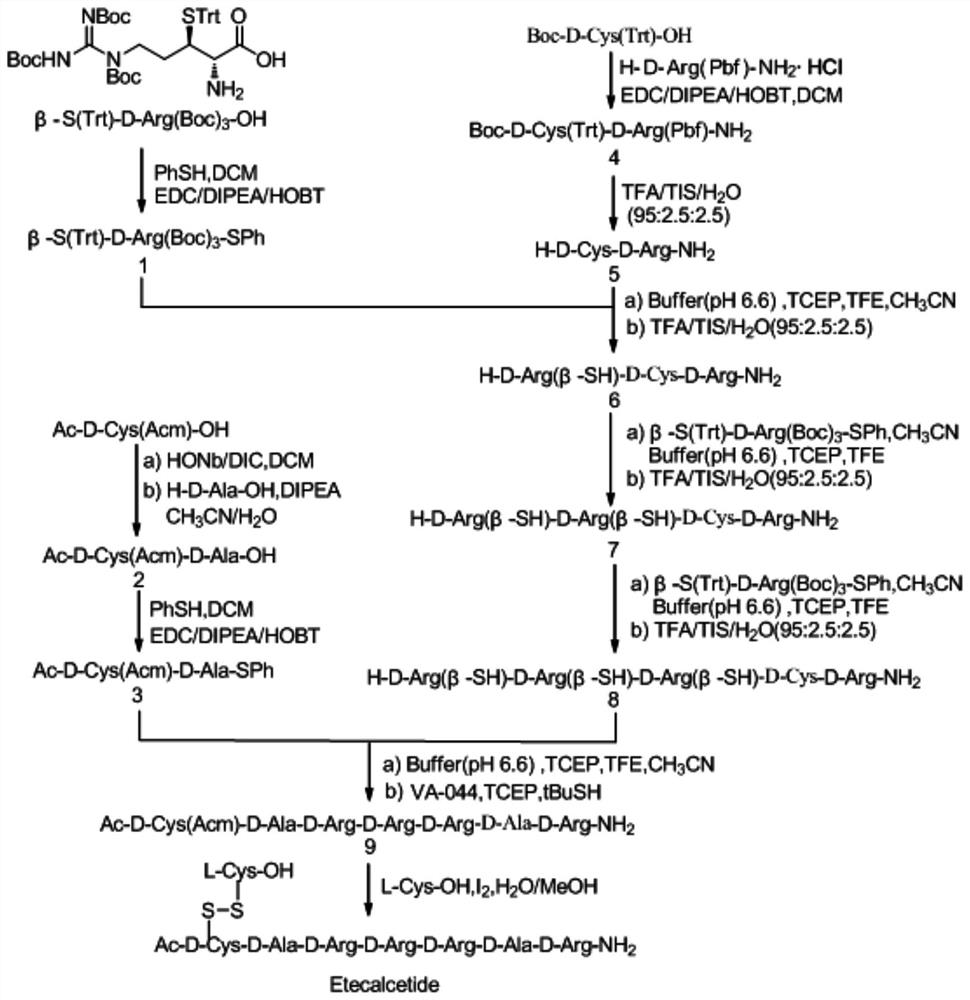
[0096] Example 1: Synthesis of Compound 1 (β-S(Trt)-D-Arg(Boc) 3 -SPh)
[0097] β-S(Trt)-D-Arg(Boc) 3 -OH (30.0g, 40mmol), PhSH (5.3g, 48mmol), EDC (13.8g, 72mmol) and HOBt (9.43g, 72mmol) were added into 300mL dichloromethane and stirred to dissolve, and cooled in an ice bath to below 5°C. Continue to add DIPEA (37.7 mL, 216 mmol) dropwise, controlling the temperature not to exceed 10°C. After the dropwise addition, stirring was continued at room temperature for 5 hours, and the reaction was monitored by TLC. After the reaction was complete, add purified water (300mL) to the reaction solution, stir for 15 minutes, leave to separate the liquids, and collect the lower organic phase. The organic phase was concentrated under reduced pressure at 30°C, the residue was stirred and dissolved with ethyl acetate (300 mL), washed successively with purified water (300 mL), saturated sodium bicarbonate solution (300 mL), and saturated sodium chloride solution (300 mL), The organic pha...
Embodiment 2
[0098] Embodiment 2: the synthesis of compound 2 (Ac-D-Cys(Acm)-D-Ala-OH)
[0099] Dissolve Ac-D-Cys(Acm)-OH (3.5g, 15mmol), HONb (3.0g, 16.5mmol) in 35mL DCM and stir to dissolve, cool in an ice bath to below 5°C, slowly add DIC (2.8mL, 18 mmol), stirring was continued at room temperature for 3 hours, and the reaction was monitored by TLC. After the reaction was complete, the reaction solution was filtered and concentrated under reduced pressure. The residue was dissolved in 35 mL of acetonitrile, 17.5 mL of purified water was added, and H-Ala-OH (1.6 g, 18 mmol) was added to continue stirring. DIPEA (4.0 mL, 22.5 mmol) was added dropwise. ). Stir at room temperature for 2 hours and monitor the reaction with TCL. After the reaction, filter and concentrate. Add 35 mL of ethyl acetate to the residue and stir to dissolve, then add saturated citric acid solution to adjust the pH to 3-4, and let the mixture stand to separate layers. The organic phase was washed with saturated ...
Embodiment 3
[0100] Example 3: Synthesis of Compound 3 (Ac-D-Cys(Acm)-D-Ala-SPh)
[0101] Ac-D-Cys(Acm)-D-Ala-OH (4.35g, 14.2mmol), H-D-Arg(Pbf)-NH HCI (1.87g, 18mmol), EDC (4.2g, 21.3mmol) and HOBt (2.88g, 21.3mmol) were added to 50mL of dichloromethane and stirred to dissolve, cooled in an ice bath to below 5°C, and continued to add DIPEA (10.9mL, 63.9mmol) dropwise, controlling the temperature not to exceed 10°C. After the dropwise addition, stirring was continued at room temperature for 5 hours, and the reaction was monitored by TLC. After the reaction was complete, purified water (50 mL) was added to the reaction liquid, stirred for 15 minutes, left to separate the liquids, and the lower organic phase was collected. The organic phase was concentrated under reduced pressure at 40°C, the residue was stirred and dissolved with ethyl acetate (50 mL), washed successively with purified water (50 mL), saturated sodium bicarbonate solution (50 mL), and saturated sodium chloride solution (50 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com