<68>Ga labeled compound for nano positron imaging agent, and preparation and application of <68>Ga labeled compound
A compound, 68ga technology, applied in the field of 68Ga-labeled compounds and their preparation, can solve the problems of low uptake rate and low targeting, and achieve the effects of good imaging effect, short reaction time and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: Ac-RVRRCK ( 68 Synthesis of Ga-NODAGA)-CBT
[0056] In this example, Ac-RVRRCK (NODAGA)-CBT was first synthesized, and the synthesis method was as follows:
[0057] Ac-RVRRCKCBT (66mg, 0.06mmol), tri-tert-butoxy-1,4,7-triazacyclononane-1,4-diacetate-7-carboxyethyl acetate (NODAGA (OtBu) 3 ) (33mg, 0.06mmol), O-benzotriazole-tetramethyluronium hexafluorophosphate (HBTU, 22.8mg, 0.06mmol), DIPEA (18μl, 0.09mmol) were dissolved in 2mL of DMF, and the reaction was stirred at room temperature Overnight, HPLC separation and purification to obtain the compound Ac-RVRRCK (NODAGA (OtBu) 3 )-CBT (25 mg, 0.015 mmol, yield: 25%). Ac-RVRRCK (NODAGA (OtBu) 3 )-CBT dissolved in CH containing 1% (v / v) triisopropylsilane, 95% (v / v) TFA 2 Cl 2 solution (3mL), stirred at room temperature for 3h, hydrolyzed the protecting group, separated and purified by HPLC to obtain the labeled precursor Ac-RVRRCK(NODAGA)-CBT (16mg, 0.011mmol, yield: 73%), according to the mass spect...
Embodiment 2
[0059] Embodiment 2: Ac-RVRRCK ( 68 Synthesis of Ga-NODAGA)-CBT
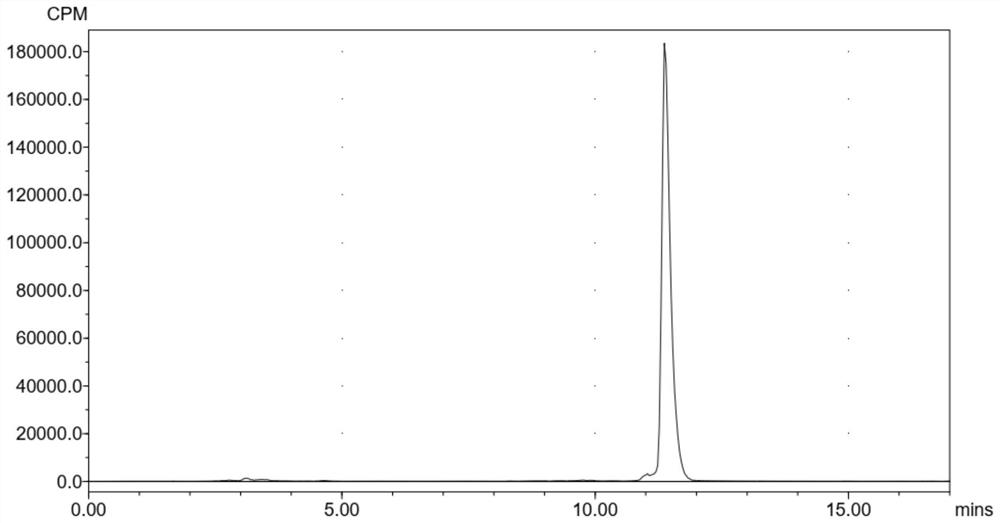
[0060] In this example, Ac-RVRRCK ( 68 The preparation method of Ga-NODAGA)-CBT is as follows: rinse with 0.05mol / LHCl 4mL solution 68 Ge- 68 Ga generator, take eluent 1.2mL (8mCi 68 GaCl 3 ), add 200 μL 0.5N sodium acetate solution to adjust the pH to 4-5. Add 10 μL of 3 mg / mL Ac-RVRRCK (NODAGA)-CBT water / acetonitrile (5:1 by volume) solution, and react at 30°C for 10 min. After the reaction, the reaction solution was collected, and the purity of the reaction solution was detected by HPLC to be 97%, without further purification. The HPLC operating parameters are the same as in Example 1.
Embodiment 3
[0061] Embodiment 3: the synthesis of Ac-RVRRCK (Ga-NODAGA)-CBT
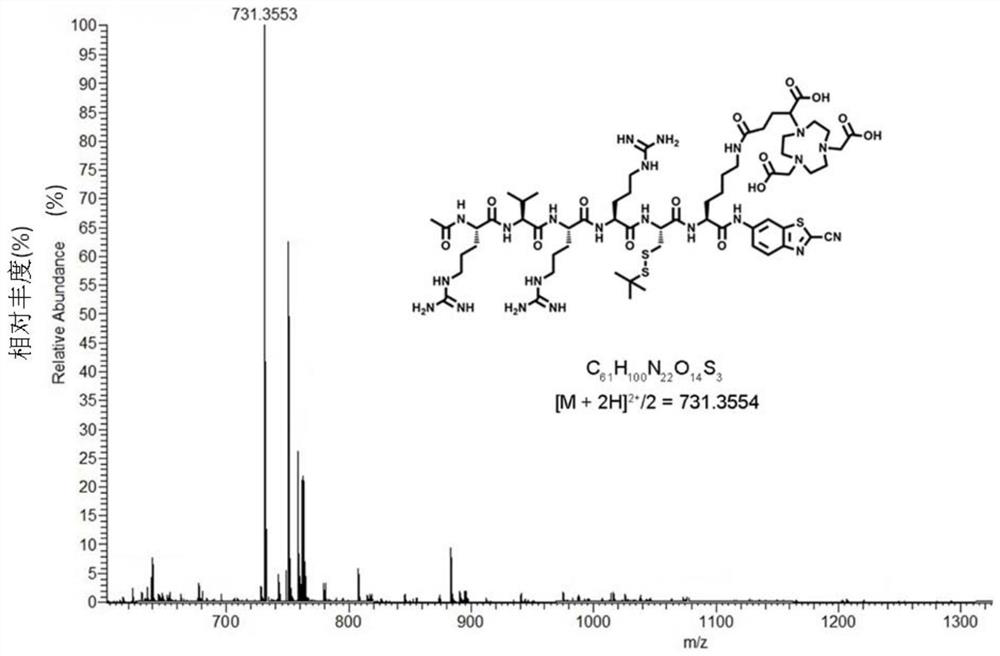
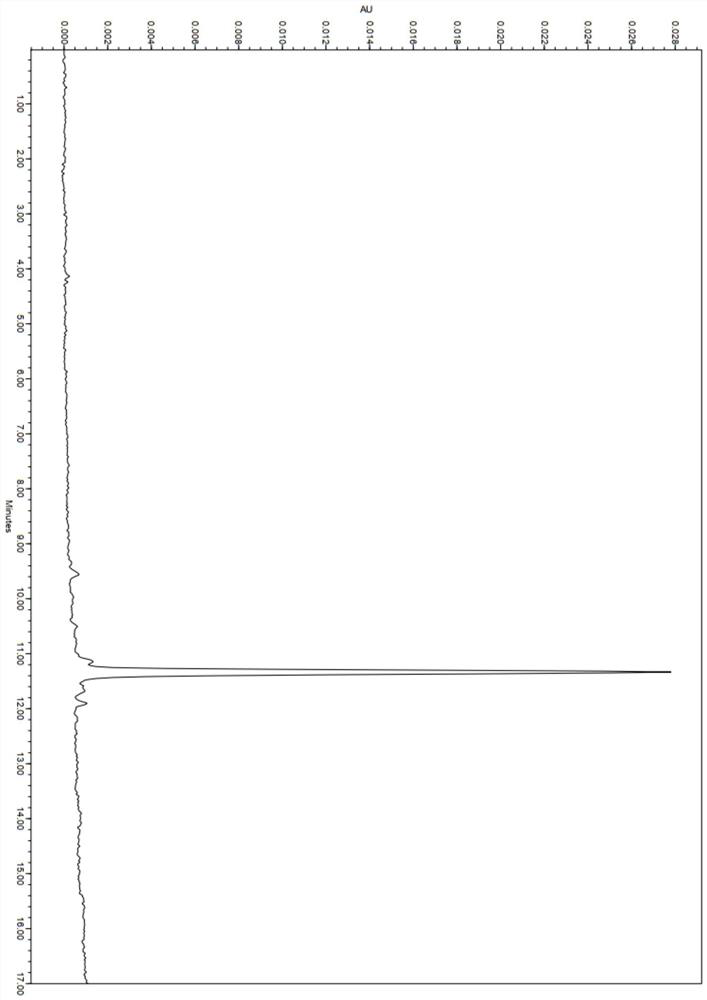
[0062] Ac-RVRRCK (NODAGA)-CBT 29mg (0.02mmoL) and 34mg GaCl 3 (0.2mmoL), dissolved in 2mL of 0.2M ammonium acetate solution (pH 5), reacted at 95°C for 25min, prepared and purified by HPLC, and lyophilized to obtain the complex product Ac-RVRRCK(Ga-NODAGA)-CBT 28mg (0.018mmol, Yield 90.0%). Product HPLC chart see image 3 , The HPLC process is detected with the ultraviolet detector in Example 1, and the ultraviolet detection wavelength is 320nm. According to the results of mass spectrometry, it can be known that the structure of the generated compound is
[0063]
[0064] [M+H] + =1527.6051, molecular formula is C 61 h 97 N 22 o 14 S 3 Ga, mass spectrum see Figure 4.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com