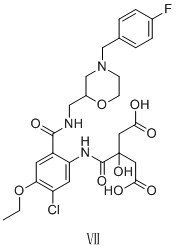
Preparation method of mosapride citrate impurity
A technology for mosapride citrate and impurities, which is applied in the field of drug synthesis and achieves the effects of strong operability, mild reaction conditions and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Preparation of compound Ⅲ: compound Ⅰ (1.00g, 4.46mmol), 2-aminomethyl-4-(4-fluorobenzyl) morpholine (formula Ⅱ) (1.06 g, 4.90mmol) EDCI (1.24g, 8.47 mmol) and HOBT (1.08g, 8.03mmol) were dissolved in DMF (30ml), and triethylamine (0.65g, 6.47mmol) was slowly added dropwise at -20°C. React at room temperature for 3 hours. After the reaction, add water (10ml) to the reaction system, and a large amount of white solid precipitates out. Suction filtration, the filter cake is purified by column chromatography to obtain 1.68g of white solid compound III with a yield of 89%.
[0022] Preparation of Compound V: Compound IV (2.00 g, 7.63 mmol) was dissolved in dry dichloromethane, oxalyl chloride (1.76 g, 13.87 mmol) was slowly added dropwise at 0°C, and the temperature was raised to room temperature to continue the reaction for 1 h. The reaction solution was concentrated to dryness to obtain compound V, which was directly used in the next step without purification.
[0023] Pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com