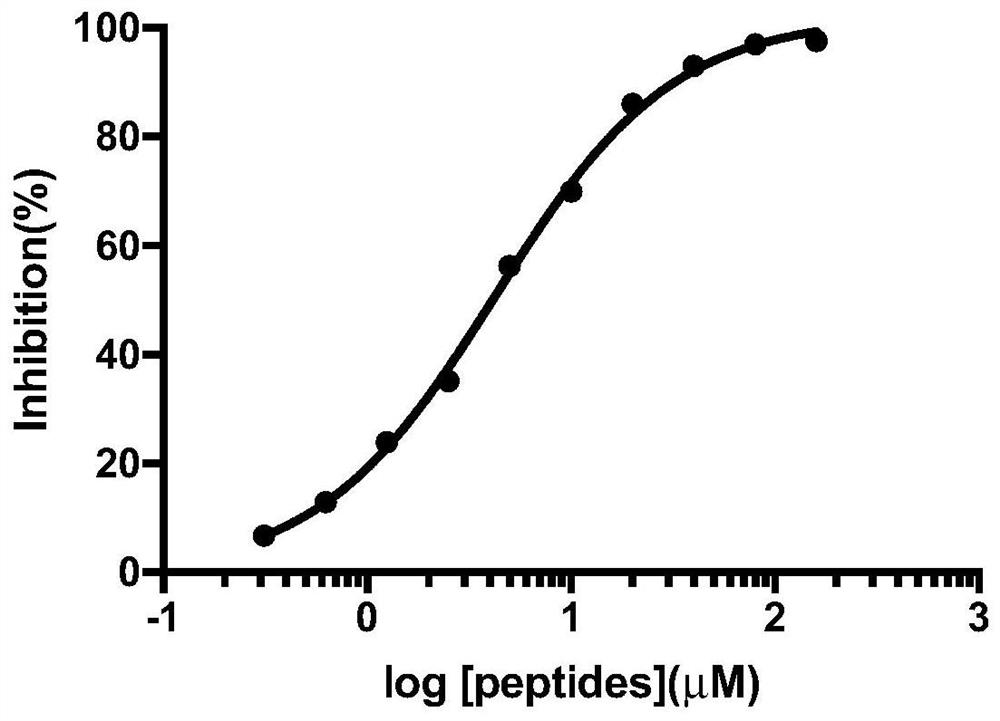
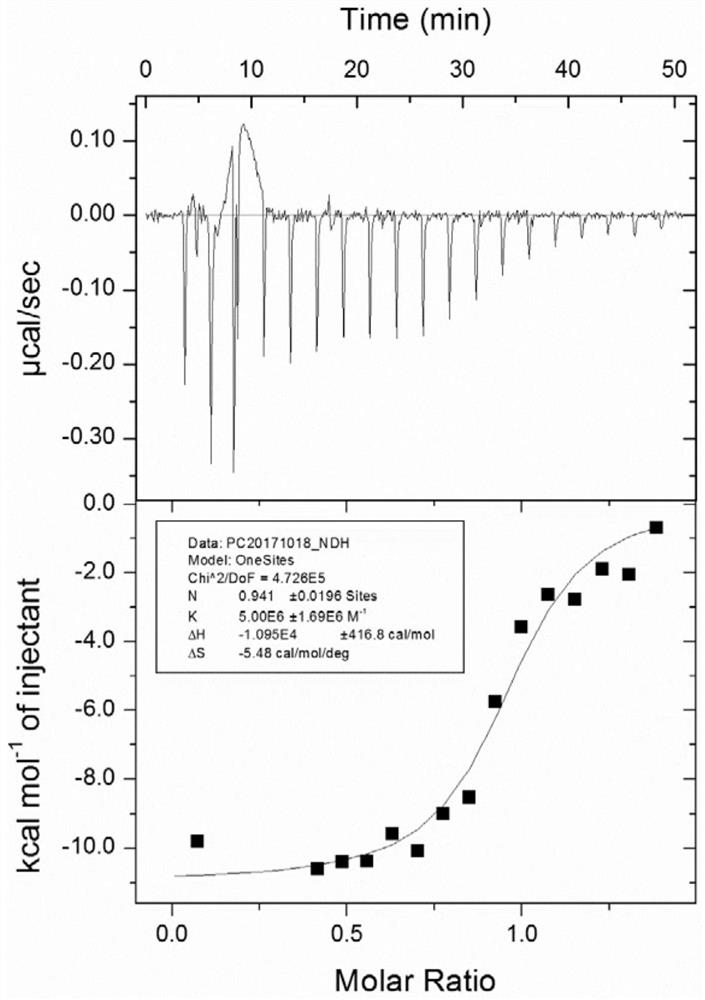
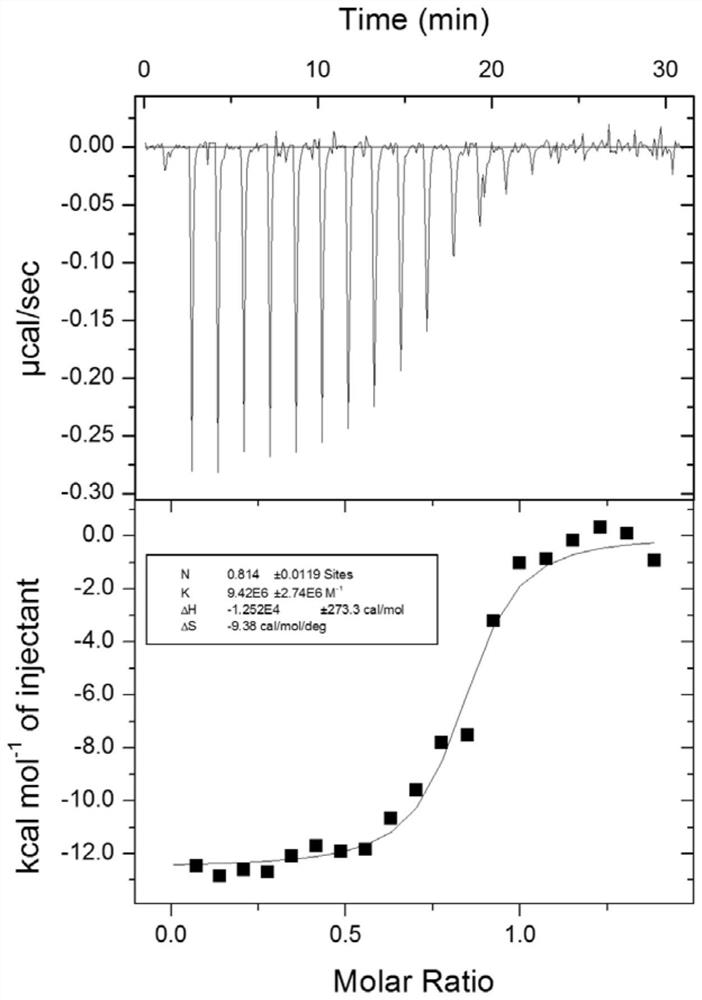
Tripeptide inhibitor for adenomatous polyposis coli/APC-stimulated guanine nucleotide exchange factor (APC/Asef) interaction and application thereof
A peptide inhibitor, P1-E-A-L-P2 technology, applied in the field of peptides, can solve the problems of APC full-length, limited application, difficult transfection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 Solid phase synthesis, cleavage, purification and identification of polypeptide 1
[0056] 1.1 Solid phase synthesis of polypeptide 1
[0057] The present invention utilizes a polypeptide synthesizer to synthesize polypeptide 1 (39901-94-5)-ESL-(1003-03-8) by a solid-phase synthesis method: using DMF as a solvent, the concentration of amino acid solutions in which various α-amino groups are protected by Fmoc 0.25mol / L, the concentration of HBTU solution and HOBt solution is 0.33mol / L, the concentration of piperidine solution is 200ml / L, and the concentration of DIEA solution is 174.2ml / L.
[0058] 1.1.1 Activated resin
[0059] Take 1g of Fmoc (fluorenebenzyloxycarbonyl)-protected butyramide resin and put it into the reactor, add an appropriate amount of dichloromethane to expand the resin, remove excess dichloromethane in the reactor by suction filtration, and put the expanded resin back into the reactor. Add 6.00mL of N,N-dimethylformamide solution contain...
Embodiment 2
[0066] Example 2 Prepare polypeptide 2(39901-94-5)-ESL-(51-67-2) according to the same method as in Example 1, the solid purity is greater than 98%, ESI: 570.57[M-H] - ;
Embodiment 3
[0067] Example 3 Polypeptide 3(31462-59-6)-ESL-(122-11-2) was prepared according to the same method as in Example 1, the solid purity was greater than 98%, ESI: 744.8[M-H] - ;
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com