Arginine modified polyethyleneimine as well as preparation method and application thereof
A technology of polyethyleneimine and arginine, which is applied in the field of chemical medicine, can solve the problems of high toxicity, PEI application limitations, and inability to meet gene therapy, etc., to achieve improved transfection efficiency, low cytotoxicity, and high gene transfection efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
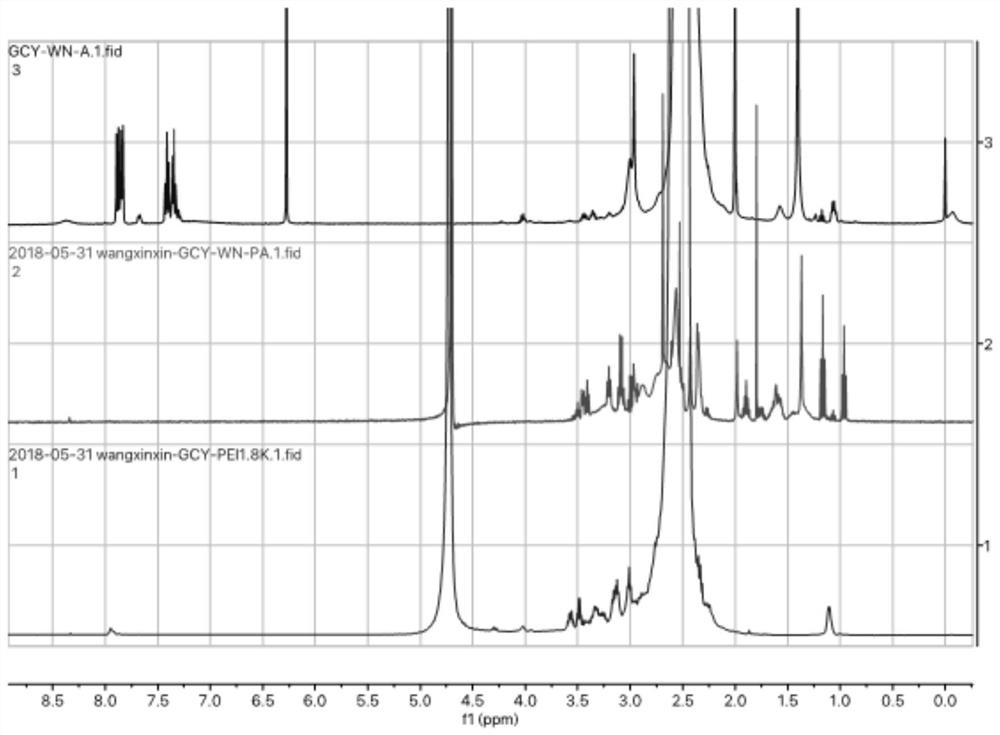
[0039] Embodiment 1, the preparation of the polyethylenimine of arginine modification of the present invention
[0040] Arginine and polyethyleneimine with a molecular weight of 1800 are prepared according to the molar ratio of arginine and primary amino groups in polyethyleneimine being 1:2. Firstly, 0.1 mol of arginine was dissolved in 9 mL of MES buffer, 0.6 mg of EDCI / NHS was added as a catalyst, and catalyzed at 25°C for 4 h, wherein the mass ratio of EDCI and NHS was 1:2; Ethyleneimine (1mol of polyethyleneimine with a molecular weight of 1800 contains 16mol of primary amino groups) and catalyzed arginine were dissolved in 12mL N-N dimethylformamide to obtain a mixed solution, and 0.5 mL of triethylamine was used as a catalyst, and then the above reaction system was placed at 25°C and stirred for 72h to carry out the substitution reaction. After the reaction, the reaction system was transferred into a dialysis bag with a molecular weight cut-off of 1000, and dialysis wa...
Embodiment 2
[0041] Embodiment 2, the preparation of the polyethylenimine of arginine modification of the present invention
[0042] Arginine and polyethyleneimine with a molecular weight of 1800 are prepared according to the molar ratio of arginine and primary amino groups in polyethyleneimine being 1:2.5. Firstly, 0.1 mol of arginine was dissolved in 9 mL of MES buffer, 0.6 mg of EDCI / NHS was added as a catalyst, and catalyzed at 25°C for 4 h, wherein the mass ratio of EDCI and NHS was 1:2; then 15.625 mmol of poly Ethyleneimine (1mol of polyethyleneimine with a molecular weight of 1800 contains 16mol of primary amino groups) and catalyzed arginine were dissolved in 12mL N-N dimethylformamide to obtain a mixed solution, and 0.5 mL of triethylamine was used as a catalyst, and then the above reaction system was placed at 25°C and stirred for 72h to carry out the substitution reaction. After the reaction, the reaction system was transferred into a dialysis bag with a molecular weight cut-o...
Embodiment 3
[0043] Embodiment 3, the preparation of the polyethylenimine of arginine modification of the present invention
[0044] Arginine and polyethyleneimine with a molecular weight of 1800 are prepared according to the molar ratio of arginine and primary amino groups in polyethyleneimine being 1:3. First, 0.1 mol of arginine was dissolved in 10 mL of MES buffer, 1.0 mg of EDCI / NHS was added as a catalyst, and catalyzed at 25°C for 4 h, wherein the mass ratio of EDCI and NHS was 1:1.5; then 18.75 mmol of poly Ethyleneimine (1mol of polyethyleneimine with a molecular weight of 1800 contains 16mol of primary amino groups) and catalyzed arginine were dissolved in 12mL N-N dimethylformamide to obtain a mixed solution, and 0.5 mL of triethylamine was used as a catalyst, and then the above reaction system was placed at 25°C and stirred for 72h to carry out the substitution reaction. After the reaction, the reaction system was transferred into a dialysis bag with a molecular weight cut-off o...
PUM
| Property | Measurement | Unit |
|---|---|---|
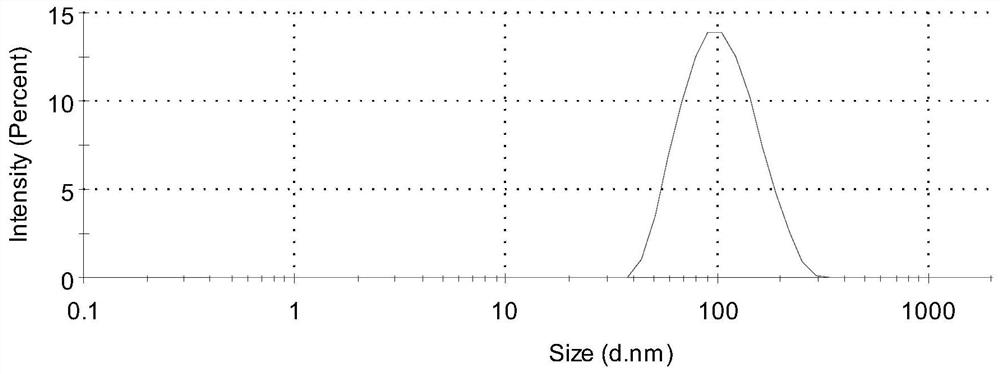
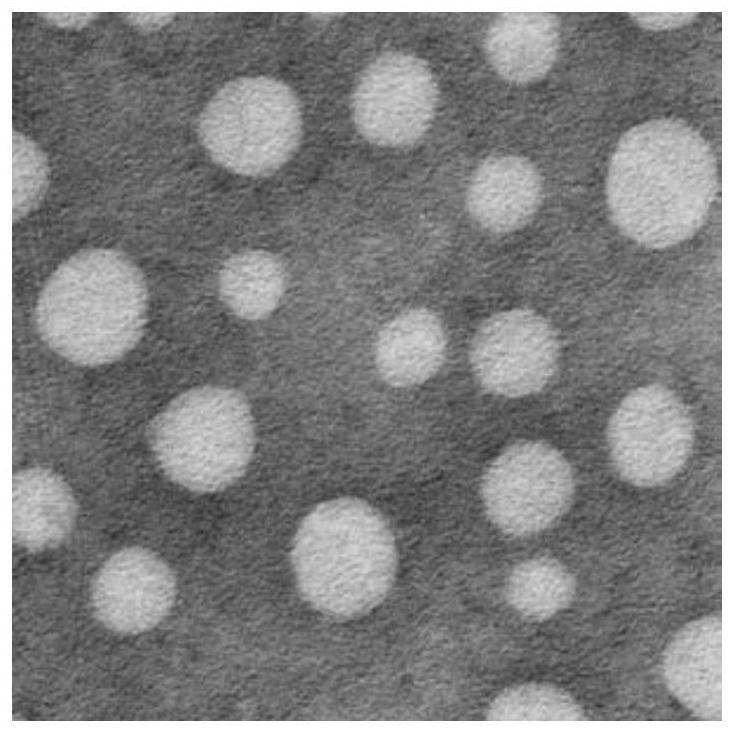
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com