Macrophage-based therapy
A technology of macrophages and cells, applied in the field of autologous macrophages and non-polarized human macrophages, which can solve the problems of macrophage function and interaction complexity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116]Example 1 Optimization of GMP-compliant monocyte culture
[0117]Isolate cells from healthy donors
[0118]The donor buffy coat, which is a source of healthy donor monocytes, is provided by the National Blood Transfusion Service of Scotland (SNBTS) Blood Donation Centre (Edinburgh, UK) under SNBTS Sample Management 13-12 and 14-02. Choose from CD14 in PBMC: Use Histopaque 1077 (Sigma) to separate PBMC from normal donor buffy coat by density centrifugation. After washing, CD14+ monocytes were separated from the mononuclear cell fraction using CliniMACS GMP grade CD14 microbeads and LS separation magnetic column (Miltenyi Biotec). Briefly, resuspend the cells in PEA buffer (phosphate buffered saline [PBS] plus 2.5 mmol / L ethylenediaminetetraacetic acid [EDTA] and human serum albumin [0.5% final volume of Alburex 20%, Octopharma]) Medium to the appropriate concentration, incubate with CliniMACS CD14 beads according to the manufacturer's instructions, then wash and pass through a magnet...
Embodiment 2
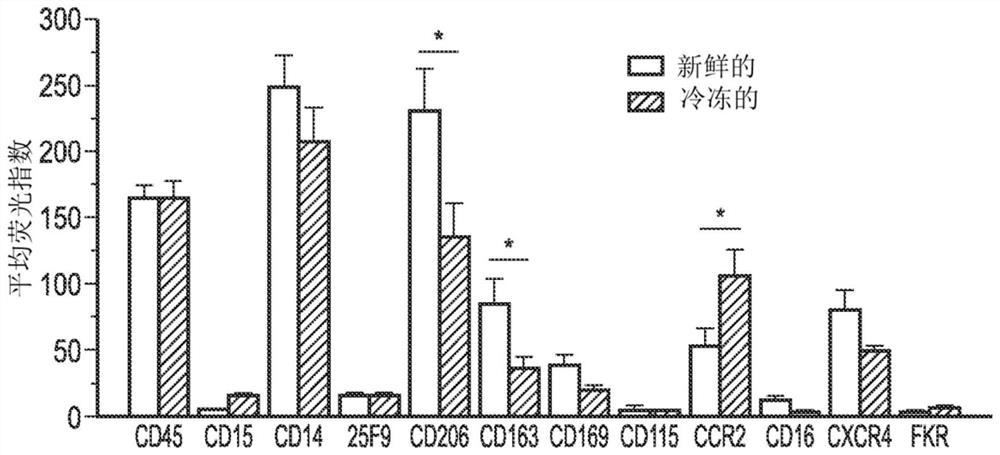
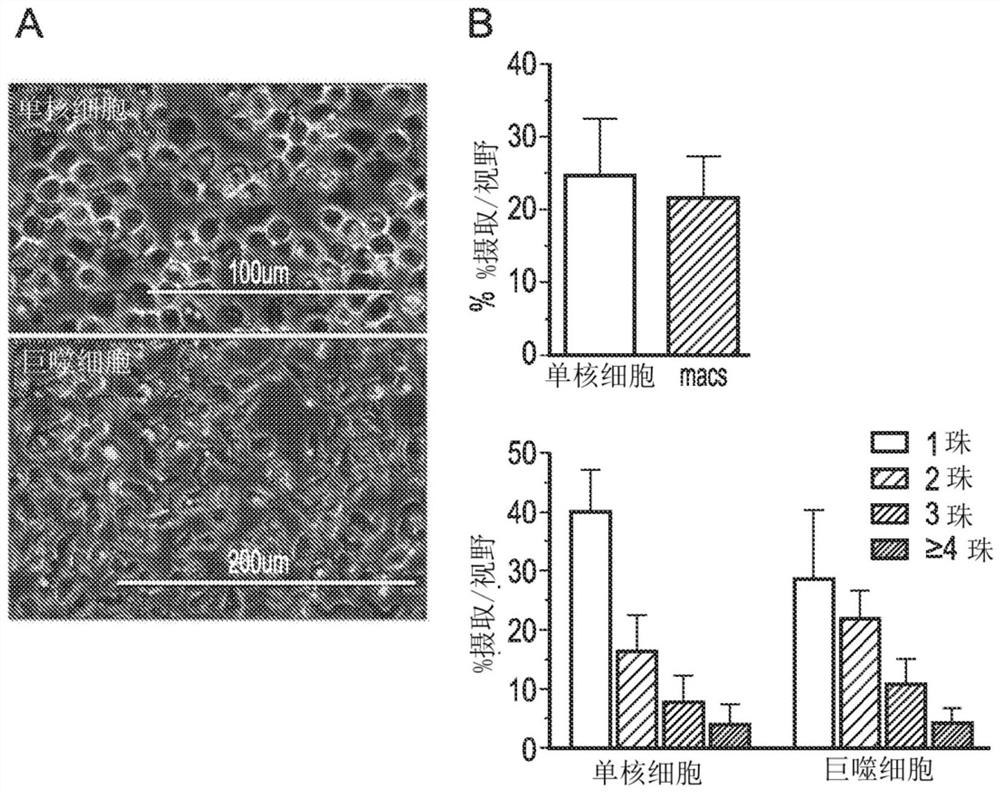
[0132]Example 2 Extended function analysis: healthy donor macrophages
[0133]Phagocytic uptake by monocytes and macrophages
[0134]The functional characterization of normal macrophages investigated the ability of cells to absorb particles. The phagocytic uptake of monocytes and macrophages from buffy coat CD14 cells was tested using pHRodo beads that fluoresce only when entering acidic endosomes. In short, monocytes or macrophages were cultured with 1-2 uL of pHRodo E. coli bioparticles (Life Technologies, Thermo Fisher) for 1 h, and then the medium was removed and the cells were washed to remove unphagocytosed particles. An EVOS microscope (Thermo Fisher) was used to assess phagocytosis, and ImageJ software (NIH free software, https: / / imagej.nih.gov / ij) was used to capture images and quantify the cellular uptake of the beads. The use of pHRodo beads in these studies can give an accurate assessment because the beads are transparent before being swallowed and will fluoresce once exposed ...
Embodiment 3
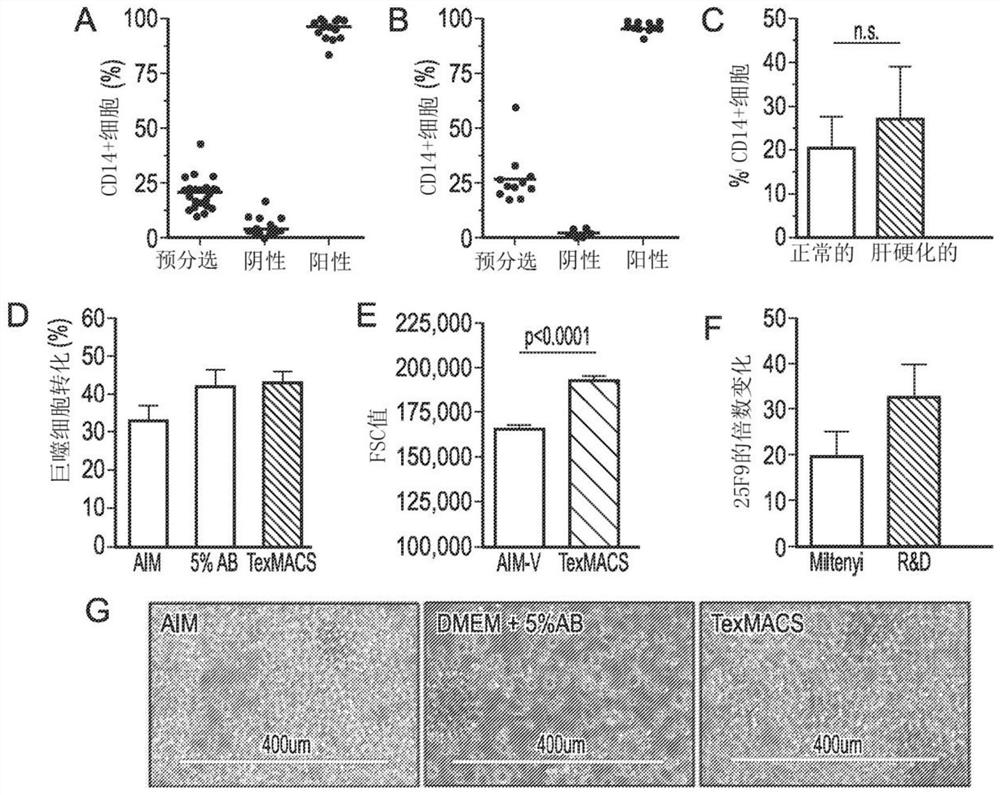
[0139]Example 3 Process Verification of Patients with Cirrhosis
[0140]Culture monocytes from patient samples
[0141]The cultured monocytes from Prodigy separation and leukopenia were measured at 2×10 per square centimeter and per milliliter.6One monocyte was cultured in a culture bag (MACS GMP differentiation bag, Miltenyi) with GMP grade TexMACS (Miltenyi) and 100 ng / mL M-CSF. Monocytes were cultured with 100ng / mL GMP-compliant recombinant human M-CSF (R&D Systems). The cells were cultured in a humidified atmosphere with 5% CO2 at 37°C for 7 days. During the culture period (day 2 and day 4), 50% volume of medium was supplemented twice, 50% of the medium was removed, and then fresh medium supplemented with 200ng / mL M-CSF (with Restore the final concentration to 100ng / mL).
[0142]Process verification
[0143]The cell culture and identification data are used to design the GMP process and then validate it. A set of markers was selected from an extensive phenotypic analysis group to be used as ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com