A strain of Escherichia coli and its whole cell catalytic production of icariin
A technology of icariin and Escherichia coli is applied in the fields of bioengineering and biosynthesis of natural compounds, and achieves the effects of high industrialization potential, simple operation and high transformation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
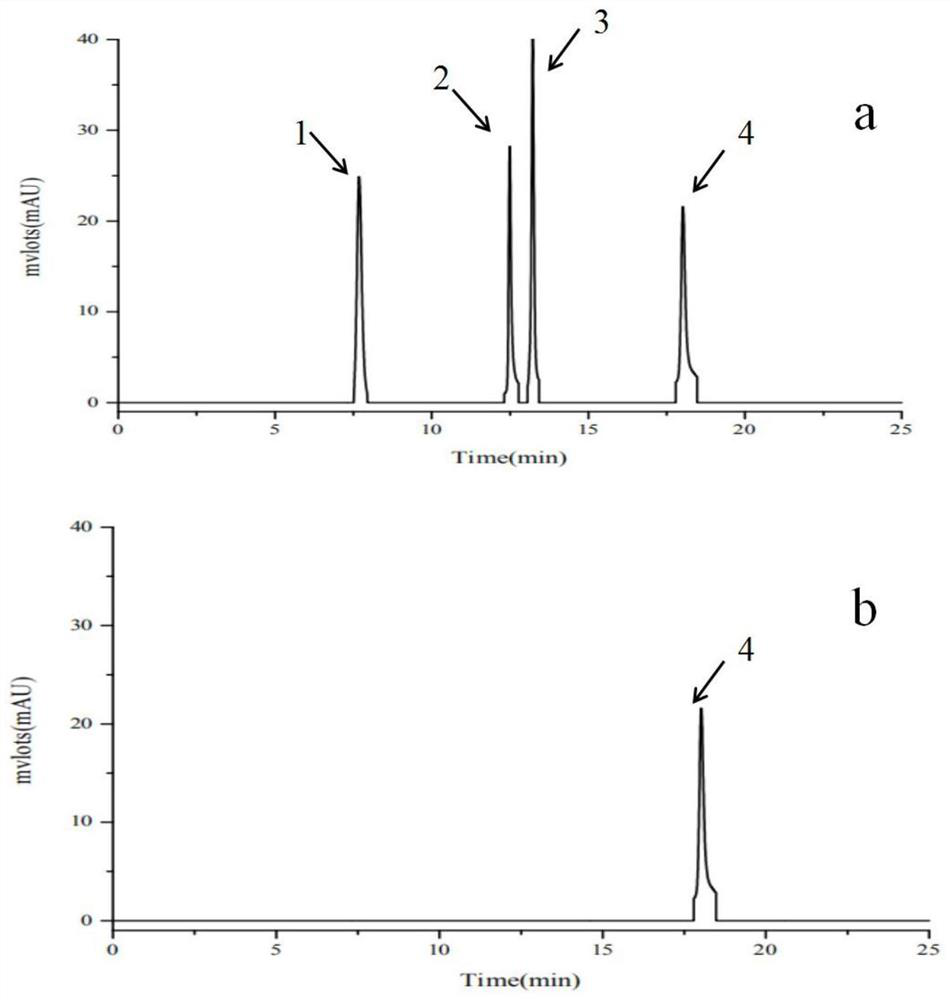
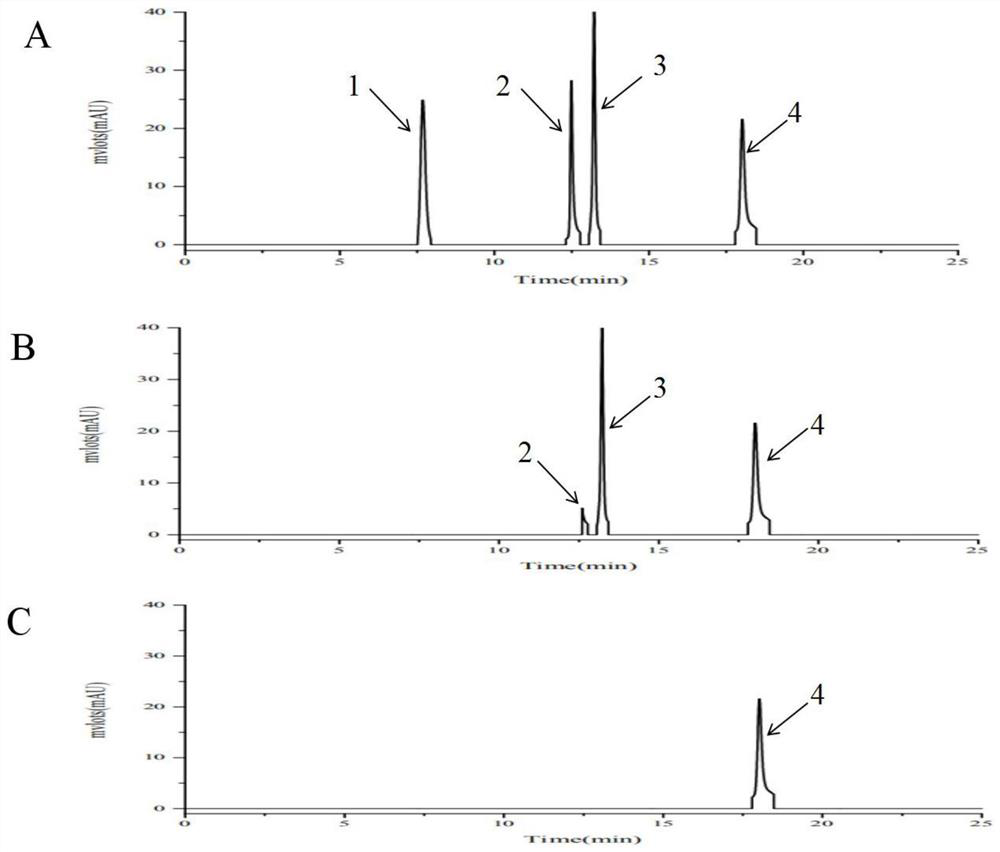
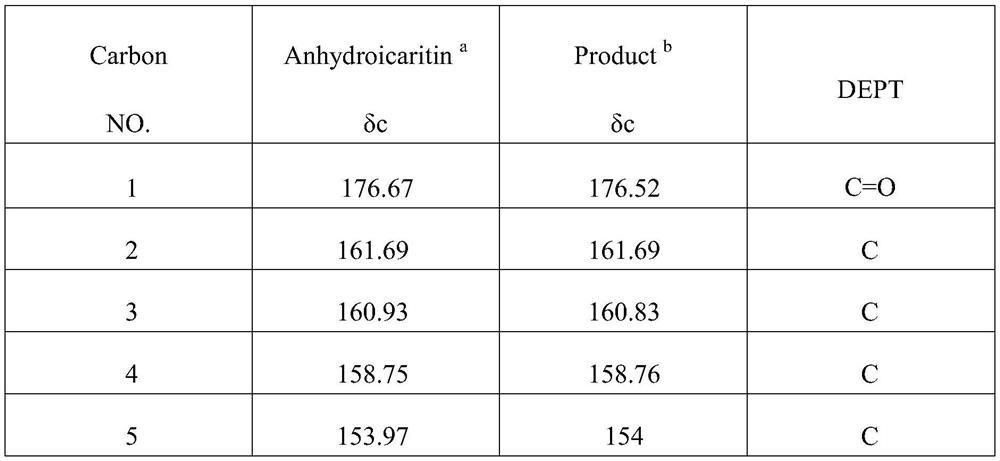
[0033] 1.1 Exploration on the ratio of the amount of the purified double-enzyme catalyzed icariin
[0034] 20°C, 0.5mM IPTG, 180rpm to induce and culture pQE-sprha2 for 20h, 30°C, 0.5mM, 200rpm IPTG to induce and culture pSE-pbgl for 8h; the induced pQE30-sprha2, pSE-pbgl cells were cytolytically purified After obtaining the pure enzyme, protein quantification of the purified pure enzyme shows that SPRHA2 is 2.2 μg / μL, PBGL is 1.2 μg / μL, and it is known that SPRHA2 reacts 0.3% (w / v) when the amount of enzyme added is 50 μg / mL. ), the conversion rate of icariin for 6h was above 95%, and the amount of enzyme added to immobilized SPRHA2 was 50 μg / mL. After diluting the two pure enzymes at appropriate times, the ratio of the amount of enzyme added (1:1, 2:1, 3: 1, 4:1, 5:1) to react 0.3% (w / v) icariin; in order to observe the reaction speed of the two enzymes during the reaction, samples were taken at 5 minutes and 5 hours of the reaction, respectively, and the heat was terminated...
Embodiment 2
[0048] Utilize Example 1 to construct the method for catalyzing icariin to generate icariin by co-expressing Escherichia coli engineering bacteria pQE-sprha2-pbgl, and the operation steps are as follows:
[0049] (1) 30° C., 0.5mM IPTG induced culture for the co-expression Escherichia coli engineering bacteria pQE-sprha2-pbgl obtained in Example 1 for 8h, after the fermentation, the cells were collected, and the wet weight of the cells was weighed;
[0050] (2) Wash 23.8 mg of wet-weight bacterial cells three times with 0.9% NaCl solution, and resuspend the bacterial cells with boric acid-borax buffer containing 0.2M boric acid and 0.05M borax, pH 8 to obtain 4.76mg / ml co-expressed Escherichia coli engineering bacteria pQE-sprha2-pbgl whole cell catalytic solution;
[0051] (3) Add 47.62 g / L Epimedium plant extract to the whole cell catalytic solution of co-expressed Escherichia coli engineering bacteria pQE-sprha2-pbgl obtained in step (2), and react at 55°C and 220 rpm for ...
Embodiment 3
[0054] Utilize Example 1 to construct the method for catalyzing icariin to generate icariin by co-expressing Escherichia coli engineering bacteria pQE-sprha2-pbgl, and the operation steps are as follows:
[0055] (1) 30° C., 0.5mM IPTG induced culture for the co-expression Escherichia coli engineering bacteria pQE-sprha2-pbgl obtained in Example 1 for 8h, after the fermentation, the cells were collected, and the wet weight of the cells was weighed;
[0056] (2) Wash 55.5 mg of wet weight cells with 0.9% NaCl solution 3 times, and resuspend the cells with boric acid-borax buffer containing 0.2 M boric acid and 0.05 M borax, pH 8 to obtain 11.1 mg / ml co-expressed Escherichia coli engineering bacteria pQE-sprha2-pbgl whole cell catalytic solution;
[0057] (3) Add 44.4 g / L Epimedium plant extract to the whole-cell catalytic solution of co-expressed Escherichia coli engineering bacteria pQE-sprha2-pbgl obtained in step (2), and react at 55°C and 220 rpm for 10 hours; The extract...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com