Application of combination of CXCL10 and HGF as pneumonia and infection source detection marker
A marker and infection source technology, applied in the field of immune detection, to achieve the effect of convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
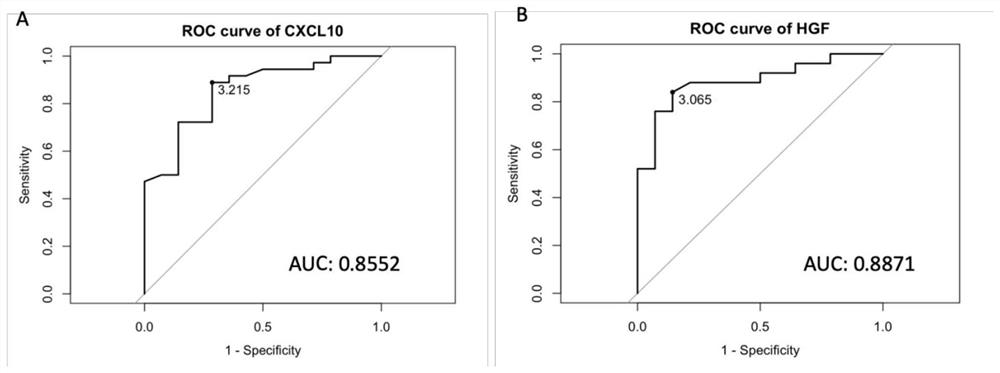
[0046] Example 1 Detection of CXCL10 and HGF gene mRNA expression levels in blood samples by real-time fluorescent quantitative PCR
[0047] Whole blood samples were collected from 20 pneumonia patients diagnosed with viral infection, bacterial infection, and virus-bacterial cross-infection and 20 normal healthy people as controls.
[0048] Featuring PureLink from Thermo Fisher Scientific TM Total RNA Blood kit (K156001) was used to extract total RNA from each blood sample according to the operating instructions. Use Thermo Scientific NanoDrop 2000 to measure the mRNA concentration in each sample, and then use Promega's GoScript TM Reverse Transcriptase (A5001) was used to synthesize cDNA, followed by The qPCR Master Mixture (A6001) of Green I dye was used to quantitatively analyze the mRNA level of myosin 1b in the blood of suspected pneumonia patients and healthy people, and the GAPDH gene was used as an internal reference gene. According to real-time fluorescence quant...
Embodiment 2
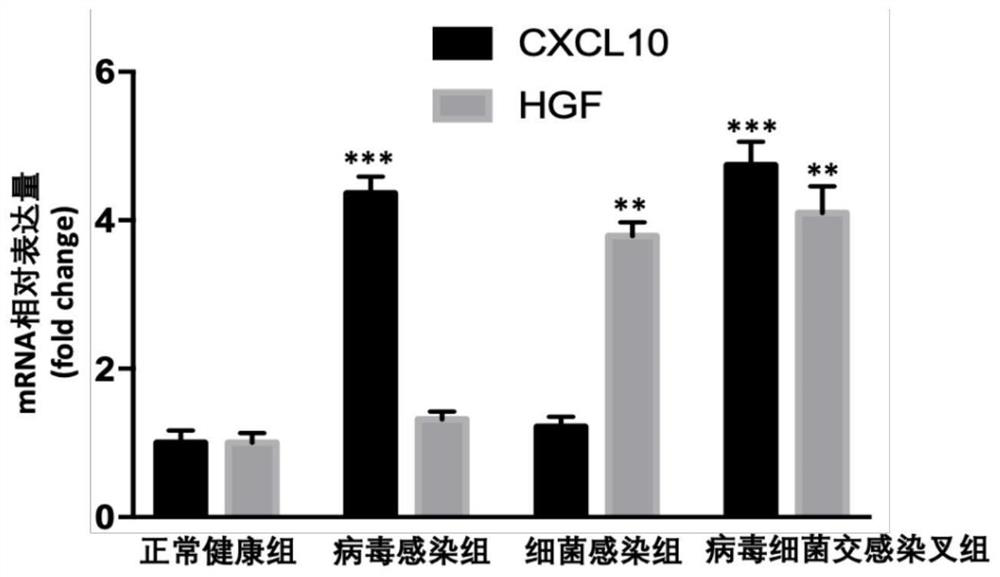
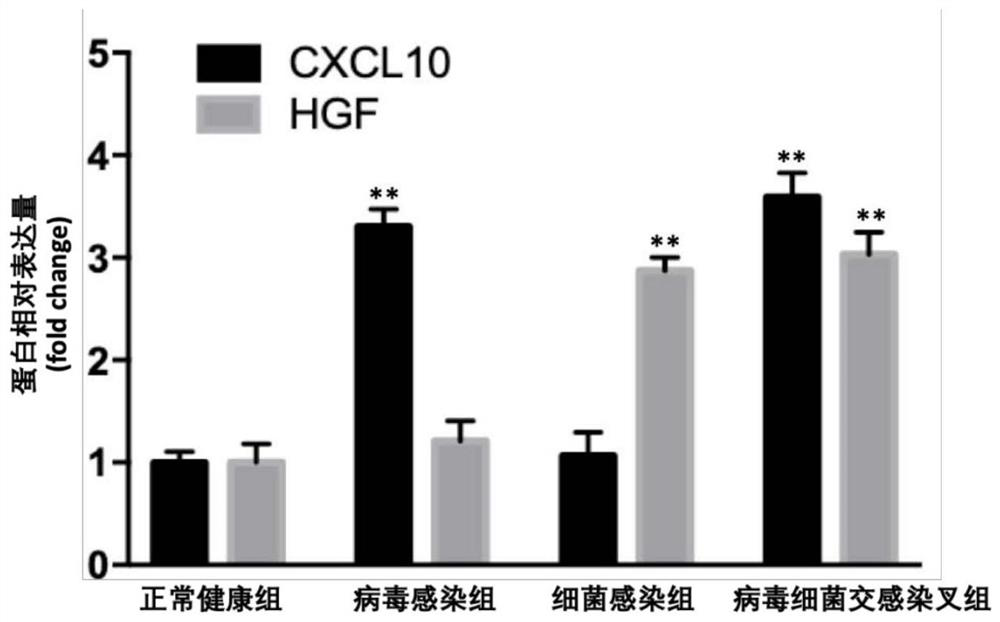
[0058] Example 2 Using enzyme-linked immunosorbent assay (ELISA) kits to detect the expression levels of CXCL10 and HGF proteins in the plasma samples of patients with pneumonia
[0059]Collect plasma samples from 20 patients diagnosed with viral infection, bacterial infection, virus and bacterial cross-infection pneumonia, and 20 normal healthy people as controls, and then use Abcam’s Human CXCL10 and HGF ELISA kits, according to the kits provided by the manufacturer. The instructions measure the CXCL10 and HGF protein content in plasma of normal healthy group, virus infected group, bacterial infected group and cross-infected virus and bacteria. Relative multiples, and do statistical analysis.
[0060] Result analysis: if image 3 As shown, compared with healthy normal people, the plasma CXCL10 in patients with pneumonia caused by viral infection was significantly increased, and HGF was not significantly changed; the plasma HGF in patients with pneumonia caused by bacterial ...
Embodiment 3
[0061] Example 3 Utilize colloidal gold test strips to detect CXCL10 and HGF biomarkers
[0062] Colloidal gold test strips based on dual detection lines of CXCL10 and HGF antibodies, each test strip contains nitrocellulose membrane, sample pad, binding pad, absorbent paper and PVC plate support, the nitrocellulose membrane package of the test strip The binding pad contains mouse anti-human CXCL10 monoclonal antibody and mouse anti-human HGF monoclonal antibody labeled with colloidal gold. Cloned antibody, so the test strip has quality control line C, CXCL10 detection line T1 and HGF detection line T2, such as Figure 4 shown. The detection operation process is as follows: before the detection, put the sample to be tested, the detection reagent and other detection materials at room temperature, take out the test card and place it flat on the table, absorb the sample to be tested with a straw, and add 2 to 3 drops of the sample to the test On the card, observe the display res...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com