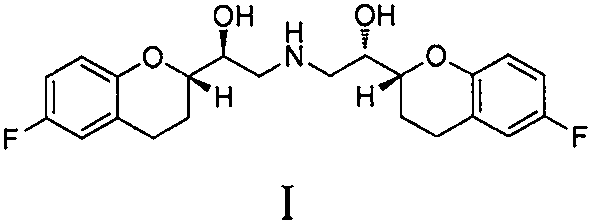
Application of nebivolol in preparation of MCR-1 enzyme inhibitor
A technology of MCR-1 and inhibitors, which is applied in the direction of resistance to vector-borne diseases, medical preparations containing active ingredients, antibacterial drugs, etc., and can solve problems such as nebivolol not seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Example 1 Effect of Nebivolol on Bacterial Growth
[0014] 1. Sample treatment: Dissolve nebivolol in DMSO, make a suitable initial concentration with culture medium, and then dilute with culture medium 2 times, a total of 4 dilutions.
[0015] 2. Bacterial suspension preparation: resuscitate BL21(DE3) and BL21(DE3) expressing mcr-1 on LB plates, pick single colonies respectively and enrich them in MH medium.
[0016] 3. Test method: put 1×10 6 c.f.u.ml -1 The bacterial solution was inoculated in a 96-well culture plate, 50 μL per well, and then 50 μL of nebivolol solution was added. The final concentration of the bacterial solution was 5×10 5 c.f.u.ml -1 , the final concentrations of nebivolol were 62.5 μM, 125 μM, 250 μM and 500 μM. At the same time, set the control, which is the same volume of control wells that only add bacterial solution. Incubate at 37°C for 16 hours, and observe with the naked eye under a dark background whether there is bacterial growth in...
Embodiment 2
[0018] Example 2 Nebivolol combined with 2 μg·mL -1 Antibacterial activity of colistin against BL21(DE3) expressing mcr-1
[0019] 1. Sample treatment: Dissolve nebivolol in DMSO, make a suitable initial concentration with culture medium, and then dilute with culture medium 2 times, a total of 4 dilutions.
[0020] 2. Bacterial suspension preparation: resuscitate BL21(DE3) expressing mcr-1 on LB plates, pick single colonies and multiply them in MH medium.
[0021] 3. Test method: put 1×10 6 c.f.u.ml -1 The bacterial solution was inoculated in a 96-well culture plate, 50 μL per well, 25 μL of nebivolol solution was added to the corresponding well, and then 25 μL of colistin solution was added. The final concentration of the bacterial solution was 5×10 5 c.f.u.ml -1 , the final concentration of nebivolol was 12.5 μM, 25 μM, 50 μM, 100 μM, and the final concentration of colistin was 2 μg·mL -1 . At the same time, a control is set, which is the control wells only added with...
Embodiment 3
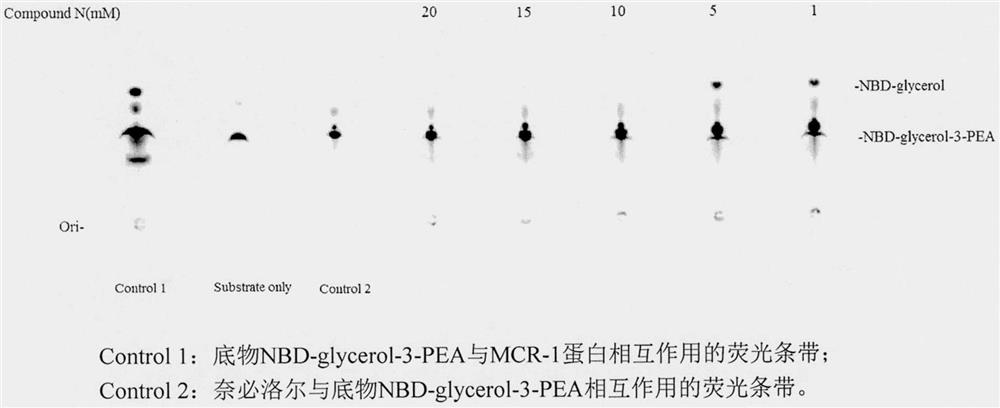
[0023] Example 3 Inhibitory effect of nebivolol on MCR-1 protein activity
[0024] 1. Construction of the MCR-1 protein expression plasmid: the mcr-1 gene was inserted into the plasmid pET21a(+) through the restriction site Nde1 / Xho1, and whether the recombinant plasmid was successfully constructed was verified by sequencing.
[0025] 2. Expression of MCR-1 protein: the recombinant plasmid pET21a(+)-mcr-1 was transfected into BL21(DE3) for bacterial culture. Pick a single colony from the agar plate and inoculate it in a medium containing 100 μg mL -1 Ampicillin was cultured overnight at 37°C in LB medium. The expanded bacteria solution was inoculated in LB culture medium at a ratio of 1:100, cultivated at 37°C until the OD600 reached 0.6, added 0.5mMIPTG, and cultivated overnight at 18°C. Centrifuge at low speed to take the precipitate, wash with 1×PBS, and store at -80°C.
[0026] 3. Purification of MCR-1 protein: Take the above-mentioned bacterial cell precipitate, resusp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com