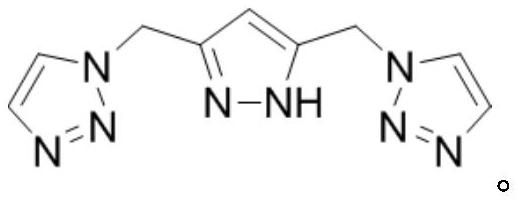
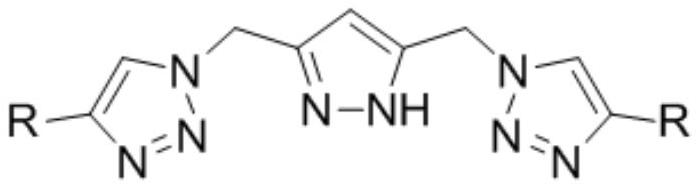
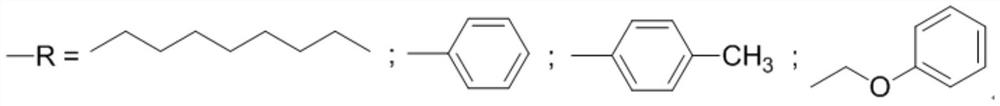
Synthesis method of dimethyl malonate
A technology of dimethyl malonate and a synthesis method is applied in chemical instruments and methods, carbon monoxide or formate reaction preparation, organic compound/hydride/coordination complex catalyst, etc., and can solve high cost and environmental pollution. , complex process and other problems, to achieve the effect of changing performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Dissolve 3.14mmol cobalt carbonyl and 3.14mmol ligand (R is phenyl) in 314mmol sulfolane, transfer to a 100mL reactor, purging the reactor three times with nitrogen, add 31.4mmol methylal, and introduce carbon monoxide to make the system pressure It was 10.0MPa, and reacted at 120°C for 12 hours. After the reaction was completed, the reactor body was fully cooled to 0°C, and the pressure was slowly released to normal pressure. The reactor was purged three times with nitrogen, and samples were taken for analysis. The experimental results are shown in Table 1.
Embodiment 2
[0030] Dissolve 4mmol cobalt carbonyl and 4mmol ligand (R is phenyl) in 314mmol sulfolane, transfer to a 100mL reaction kettle, purge the reaction kettle three times with nitrogen, add 31.4mmol methylal, and pass in carbon monoxide to make the system pressure 10.0 MPa, react at 120°C for 12 hours. After the reaction was completed, the reactor body was fully cooled to 0°C, and the pressure was slowly released to normal pressure. The reactor was purged three times with nitrogen, and samples were taken for analysis. The experimental results are shown in Table 1.
Embodiment 3
[0032] Dissolve 6.28mmol cobalt carbonyl and 6.28mmol ligand (R is phenyl) in 314mmol sulfolane, transfer to a 100mL reactor, purging the reactor three times with nitrogen, add 31.4mmol methylal, and introduce carbon monoxide to make the system pressure It was 10.0MPa, and reacted at 120°C for 12 hours. After the reaction was completed, the reactor body was fully cooled to 0°C, and the pressure was slowly released to normal pressure. The reactor was purged three times with nitrogen, and samples were taken for analysis. The experimental results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com