Culture method for colorectal cancer organoids and culture solution
An organ culture, colorectal cancer technology, applied in the culture process, tissue culture, 3D culture and other directions, can solve the problems of lack of widespread use, technical difficulty, complicated operation, etc., to reduce the tedious operation, reduce the risk of pollution, operation easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] This embodiment provides L-WRN CRL-3276 TM The preparation of the conditioned medium of the cells self-produced Wnt-3a, R-Spondin1 and Noggin three cytokines, including:
[0075] 1) Thaw low-passage L-WRN cells, culture them in conventional DMEM medium containing 10% FBS for one day, and then replace with conventional medium adding G418 (500 μg / mL) and hygromycin (500 μg / mL) until confluence.
[0076] 2) Digest with TE at 37°C for 3-5 minutes, resuspend with regular culture medium, and passage ratio is 1 / 5.
[0077] 3) After culturing for 3-4 days until confluence, the medium was replaced with primary medium (Advanced DMEM / F12+20% FBS+2mM GlutaMAX+1% double antibody).
[0078] 4) After culturing for 24 hours, collect the supernatant for the first time, and centrifuge at 2000g for 5 minutes. After collecting the supernatant, continue culturing with the same volume of primary culture medium, and collect the supernatant every 24 hours. The supernatants of the second, thi...
Embodiment 2
[0081] 1. In vitro processing of colorectal cancer biopsy tissue
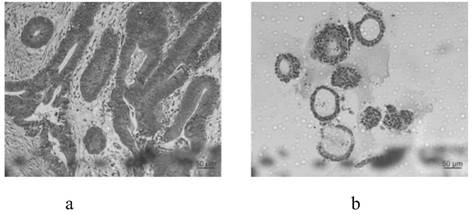
[0082] 1) The tumor tissue (about 0.5 g) of the protruding solid part of the top of the colorectal cancer tumor was excised at multiple points. Cryomedium is preserved for delivery to the laboratory. The inventors found in multiple cultures that when taking about 0.5 g of tumor tissue / block, a sufficient amount of single cells can be obtained without wasting a large amount of digestive juice.
[0083] 2) Wash with ice-cold 1xPBS containing antibiotics (1% double antibody (penicillin-streptomycin) + 100ug / ml primocin + 50ug / ml getamicin) up and down for about ten times until the supernatant is clear.
[0084] 3) Place the tissue on ice and shear until there are no visible particles.
[0085] The digestion solution was made into 1mg / mL collagenase IV, 100ug / mL DNAse, and 100ug / ml hyaluronidase digestion working solution. Filter through a 0.22um sterile filter and preheat to 37°C before use. Add 10mL of digest...
Embodiment 3
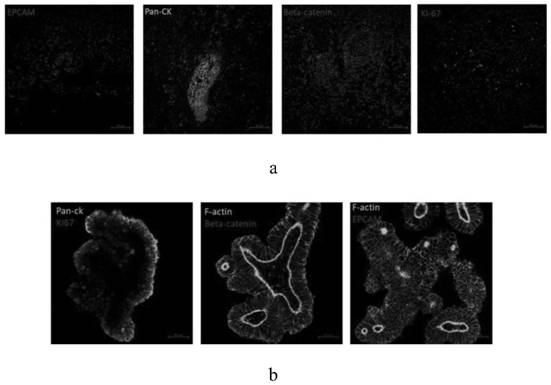
[0095] Cryopreservation of colorectal cancer organoids
[0096] 1) Collect organoids grown after five days of growth within a certain generation, add cell recovery solution and place on ice for 30 minutes to melt Matrigel.
[0097] 2) Centrifuge, discard the supernatant, resuspend the pellet with 500 μL of Tryple, place it at 37° C. for 3 minutes, and add culture medium to stop the digestion.
[0098] 3) Centrifuge again and discard the supernatant. According to the amount of organoids in one well of a six-well plate, add 1 mL of cryopreservation solution for cryopreservation, about 500,000 organoid fragments / 1 mL of cryopreservation solution, and the component of the cryopreservation solution is FBS containing 10% DMSO. Cryopreservation uses a programmed cryopreservation gradient cooling method.
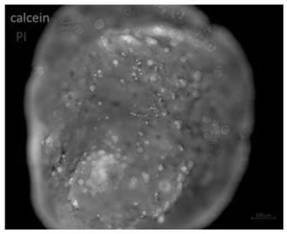
[0099] see results Figure 7 ,From Figure 7 It can be seen that organoids can still grow stably after cryopreservation and rethawing without affecting cell viability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com