Method for preparing chiral tert-butyl sulfinamide
A technology of tert-butylsulfinamide and chirality, which is applied in the field of preparation of tert-butylsulfinamide, can solve the problems of high cost, long overall steps, unfavorable industrial production, etc., and achieve the effect of cheap price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
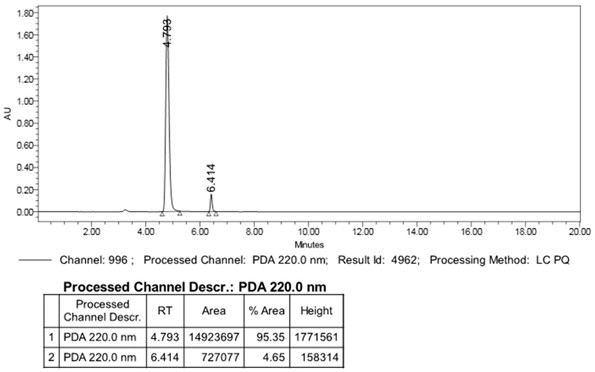
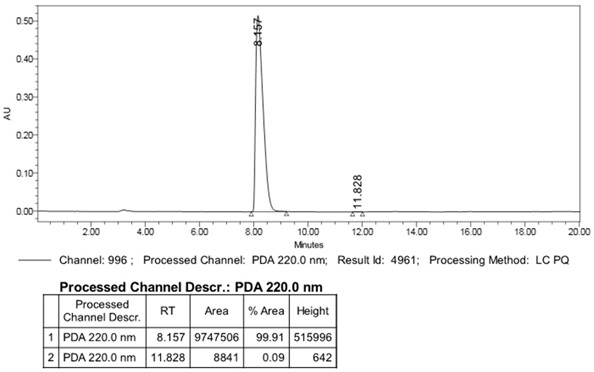
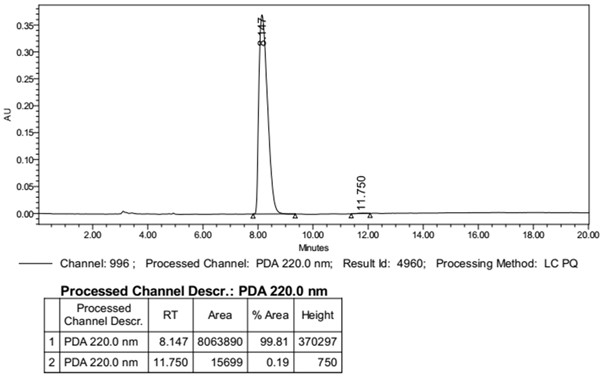
Image
Examples
Embodiment 1
[0038] Preparation of Compound 2
[0039]
[0040] In a dry glass reaction kettle under nitrogen protection, magnesium chips (24g, 1.0eq) were added and heated to 45-55°C. Take another preparation bottle, add tert-butyl bromide (177g, 1.3eq) and 2-methyltetrahydrofuran MeTHF (500g), mix well, add part of the mixture dropwise into the reaction bottle to form a Grignard reaction system, and cool down to 40 -50°C, drop the mixed solution of tert-butyl bromide, after the dropwise addition, stir the reaction for more than 5 hours, cool down the Grignard reaction solution to -5 to 5°C, feed sulfur dioxide into the reaction kettle, the sulfur dioxide The weight is (171g, 2.5eq). After insulated reaction at around 0°C for 1 hour, add concentrated sulfuric acid dropwise to the reaction kettle to quench the reaction, distill under reduced pressure, control the temperature at 30-45°C, distill under reduced pressure to dry to viscous liquid (remove excess sulfur dioxide), then add Te...
Embodiment 2
[0054] Preparation of Compound 2
[0055]
[0056] In a dry glass reaction kettle under nitrogen protection, add magnesium chips (10g, 1.0eq), heat to 45-55°C, take another preparation bottle, add tert-butyl iodide (98g, 1.3eq), methyl tert-butyl Ether (300g), mix well, drop the mixed solution into the reaction flask to form a Grignard reaction system, cool down to 40-50°C, continue to drop the mixed solution of tert-butyl bromide, after the dropwise addition, stir and react for more than 5h , the temperature of the Grignard reaction solution was lowered to -5 to 5°C, and sulfur dioxide was introduced into the reaction kettle, and the weight of the sulfur dioxide introduced was (60g, 2.3eq). After insulated reaction at around 0°C for 1 hour, add concentrated sulfuric acid dropwise to the reaction kettle to quench the reaction, distill under reduced pressure, control the temperature at 30-45°C, distill under reduced pressure to dry to viscous liquid (remove excess sulfur dio...
Embodiment 3
[0070] In this example, other steps are the same as in Example 1, the difference is that compound 4-3 is prepared by the following method.
[0071] Preparation of compound 4-3 (isobutyl ester)
[0072]
[0073] Dissolve 2g (14.2 mmol, 1.0eq) of compound 3 in 6ml of methyl tert-butyl ether for use; in a 200mL three-necked flask, add 3.2g of isobutanol (42.6 mmol, 3.0eq), 5.07g of quinidine g (15.6 mmol, 1.1 eq) and 60ml methyl tert-butyl ether, under nitrogen protection, lower the temperature to -50°C±5°C, add the methyl tert-butyl ether solution of compound 3 dropwise, and control the temperature at - Below 30°C (±5°C); after dripping, keep the reaction at -35°C for 2 hours. It is found that the reaction system becomes viscous and cannot continue to stir. The temperature of the reaction system was raised to -25°C, and the reaction was continued for two hours, and the reaction of the raw materials was detected to be complete.
[0074] Post-treatment: add ice water, add 10%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com