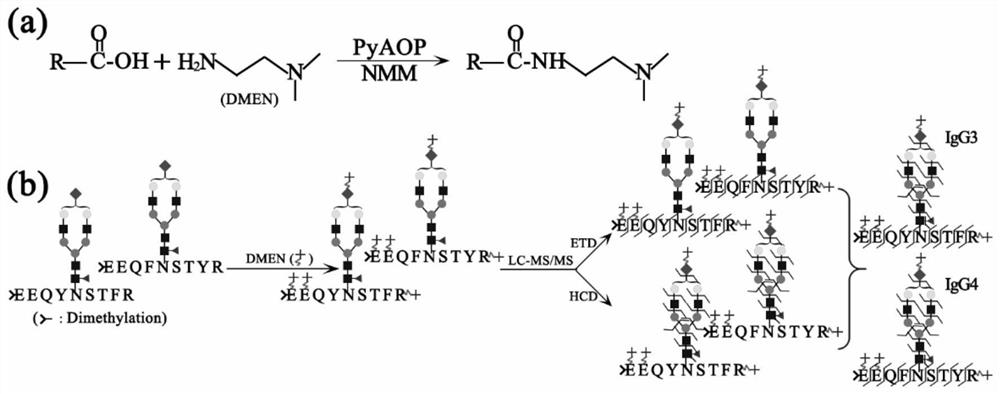
Method for complete glycopeptide derivation and charge transfer fragmentation mass spectrometry
A technology of charge transfer and mass spectrometry, which is applied in the field of glycoproteomics analysis, can solve problems such as fragmentation, limit the application of ETD, and invalid ETD, and achieve the effect of increasing the number of charges and improving the efficiency of fragmentation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
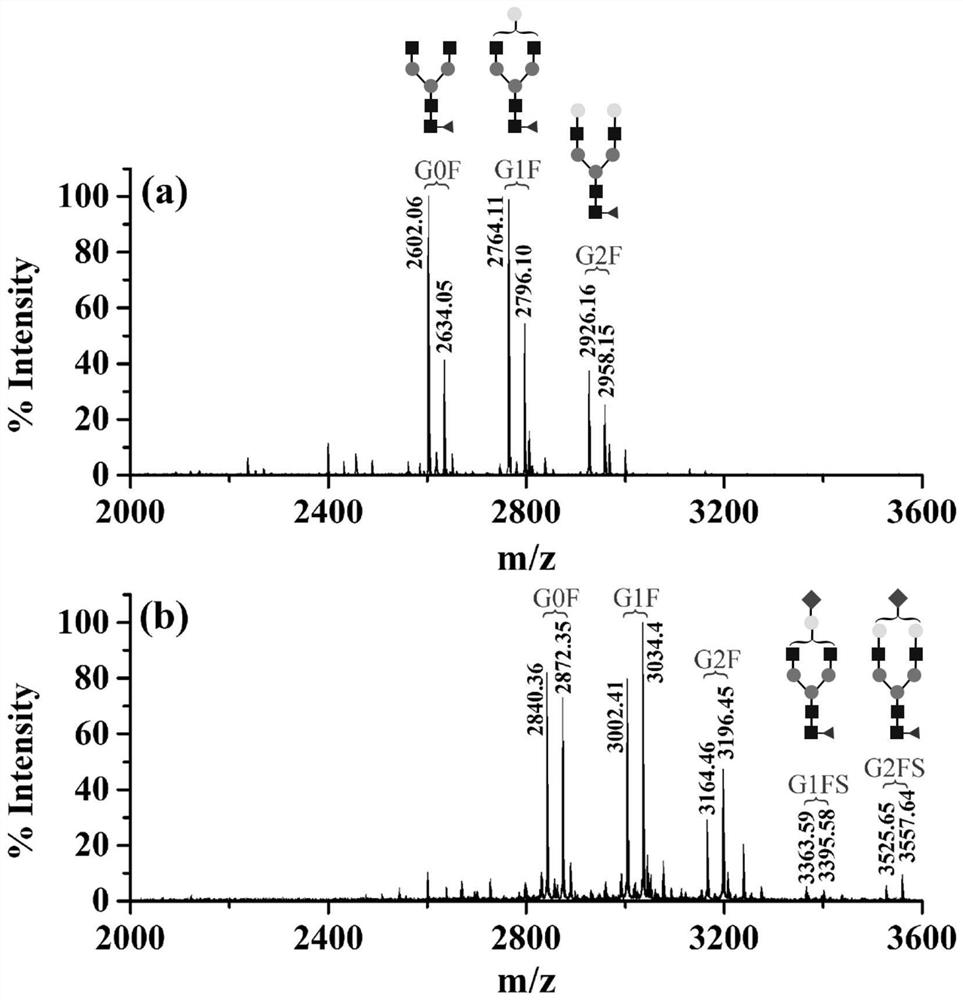
[0051] DMEN derivatization experiment of complete glycopeptide of embodiment 1
[0052] Dissolve 100 μg of IgG protein with DMSO, then add 1 μL 1M TEAB, 5 μL 5M DMEN, and 2.5 μL NMM in sequence, mix them evenly, and then add 12.5 μL 400 mM PyAOP solution (dissolved in DMSO). After thorough mixing, the reaction solution was suspended at room temperature for 2 h. After the reaction, add 800 μL ACN / HO 2 O / TFA (80 / 19 / 1, v / v / v) solution, after mixing, carry out ZIC-HILIC enrichment. MALDI-TOF-MS analysis was carried out on the target of the enriched solution (the final solution containing IgG complete glycopeptides), and the results were as follows figure 2 shown.
Embodiment 2
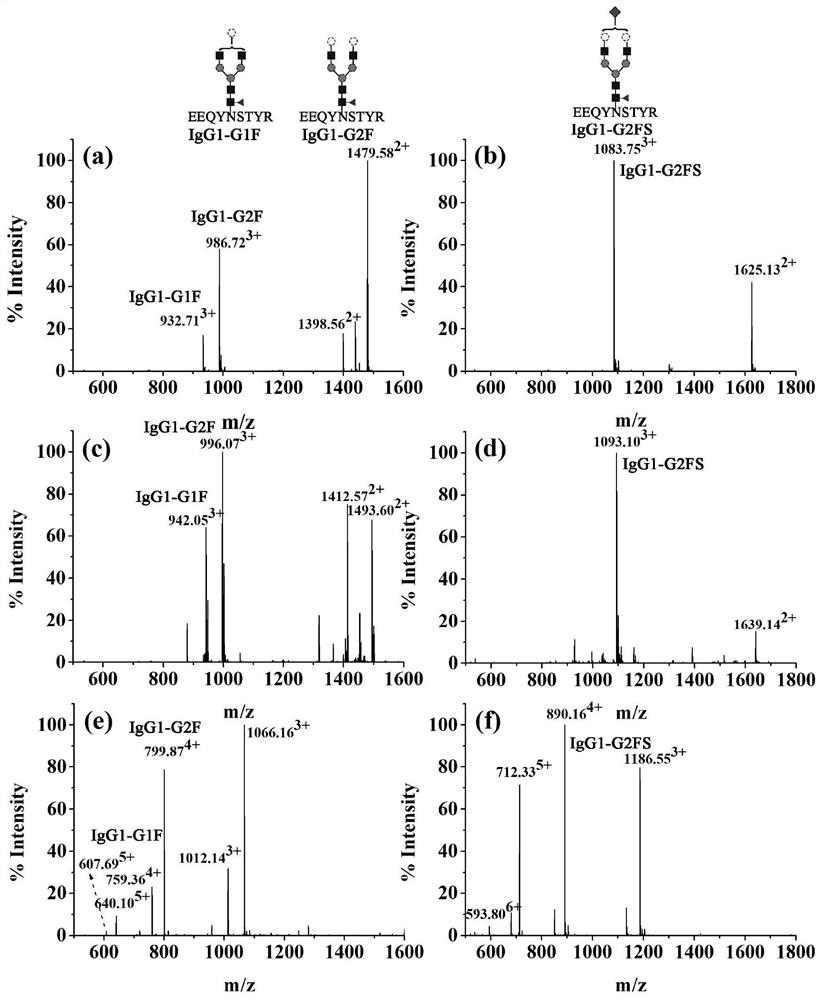
[0053] Example 2 Experiment of increasing charge number in DMEN-derived complete glycopeptide electrospray mass spectrometry
[0054] 100 µg of IgG protein was dissolved with DMSO. Then 1 μL of 1M TEAB, 5 μL of 5M DMEN, and 2.5 μL of NMM were added in sequence, and mixed evenly, and then 12.5 μL of 400 mM PyAOP solution (dissolved in DMSO) was added. After thorough mixing, the reaction solution was suspended at room temperature for 2 h. After the reaction, add 800 μL ACN / HO 2 O / TFA (80 / 19 / 1, v / v / v) solution, mixed well and enriched by ZIC-HILIC, the enriched solution was lyophilized, and then analyzed and detected by LC-ESI-MS using ETD mode ;results such as image 3 shown.
Embodiment 3
[0055] Example 3 DMEN-derived complete glycopeptide experiment to improve the efficiency of mass spectrometry charge transfer fragmentation
[0056] 1. Dissolve 100 μg of standard IgG protein (IgG) with DMSO. Then 1 μL of 1M TEAB, 5 μL of 5MDMEN, and 2.5 μL of NMM were added in sequence, and mixed evenly, and then 12.5 μL of 400 mM PyAOP solution (dissolved in DMSO) was added. After thorough mixing, the reaction solution was suspended at room temperature for 2 h. After the reaction, add 800 μL of ACN / H2O / TFA (80 / 19 / 1, v / v / v) solution, mix well and carry out ZIC-HILIC enrichment, freeze-dry the solution and then use LC-ESI-MS, using ETD-MSMS mode analysis detection; results such as Figure 4 shown.
[0057] 2. Dissolve 100 μg Fetuin with DMSO. Then 1 μL of 1M TEAB, 5 μL of 5M DMEN, and 2.5 μL of NMM were added in sequence, and mixed evenly, and then 12.5 μL of 400 mM PyAOP solution (dissolved in DMSO) was added. After thorough mixing, the reaction solution was suspended at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com