Lymphohematopoietic engineering using cas9 base editors
A base editing and lymphatic technology, applied in genetic engineering, genetically modified cells, blood/immune system cells, etc., can solve the problem of low efficiency of a single nucleotide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
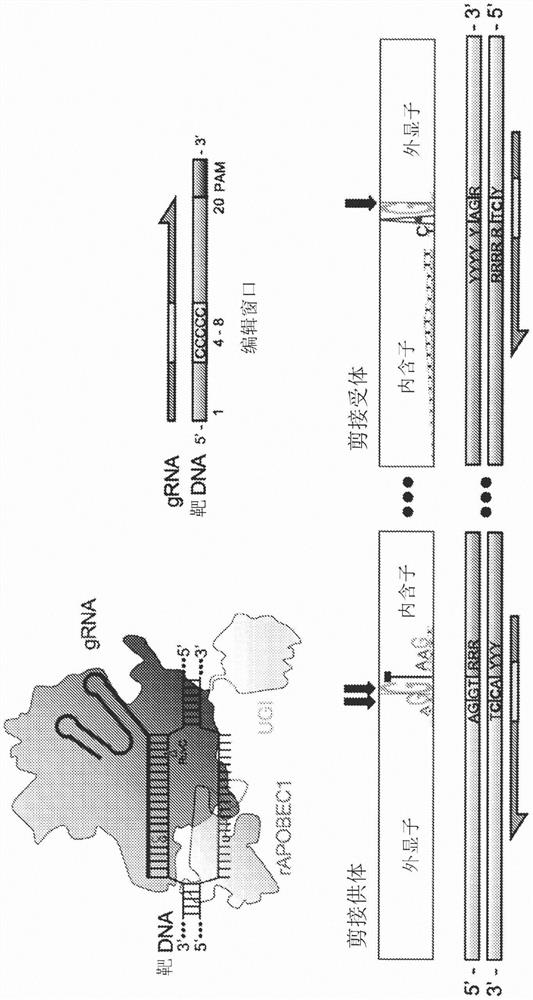
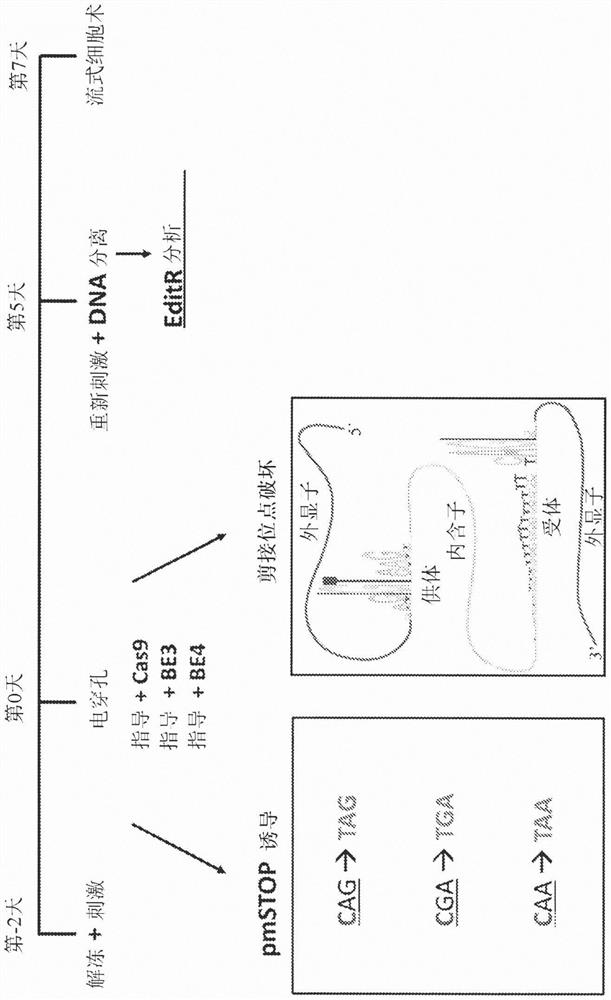
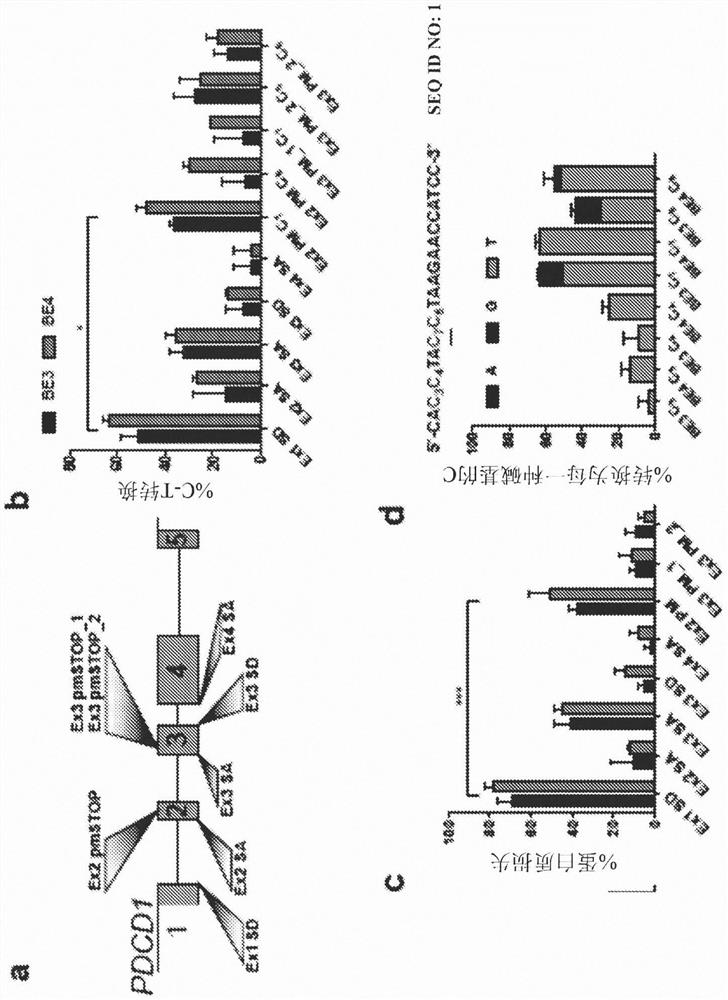
[0067] Example 1 - Splice site base editing
[0068] Base editing has previously been used to induce premature stop (pmSTOP) codons for gene knockout in mouse and mammalian cells 15-18 . However, we believe that splice site disruption has several advantages over induction of the pmSTOP codon ( figure 1 ). For example, stop codon readthrough has been shown to occur at frequencies as high as 31% in some genes and can be promoted under cellular stress conditions 19,20 . Splice site editing alleviates this concern because it alters gene processing at the RNA level 21 , which is unlikely to be bypassed at the translation level. Furthermore, current base editors cannot produce strict C-T edits, and even the latest base editors produce up to 25% off-target edits (C-G / A) 22 . In the case of pmSTOP, off-target editing prevents premature stop codon formation, thereby reducing the efficiency of protein knockdown and instead generating potentially undesired amino acid changes.
...
Embodiment 2
[0114] Example 2 - Base Editing in Natural Killer (NK) Cells
[0115] method
[0116] Isolation of Peripheral Blood Mononuclear Cells (PBMC): Peripheral blood was diluted 3:1 with chilled IX PBS. Diluted blood was dropped onto 15 mL of Lymphoprep (Stem Cell Technologies). Centrifuge cells at 400 x g for 25 min without brake. The buffy coat was removed and washed with cold 1X PBS and centrifuged at 400 x g for 10 min. The supernatant was removed and cells were either frozen as PBMCs or used immediately for purification of NK cells.
[0117] CD3 - CD56 + Isolation of NK cells: adjust the density of PBMCs to 5 x 10 7 cells / mL and transfer the cells to a 14 mL polystyrene round bottom tube. NK cells were isolated using Human NK Cell Enrichment Kit or Human NK Cell Isolation Kit (Stem Cell Technologies) according to the kit instructions. Enriched cells were counted and analyzed for purity (%CD56+, %CD3+) by flow cytometry.
[0118] Stimulation of CD3-CD56+ NK cells: CD3-...
Embodiment 3-CD34
[0137] Example 3 - Base editing in CD34+ hematopoietic stem-progenitor cells (HSPC)
[0138] Materials and methods
[0139]Medium: StemSpan Serum Free Expansion Media II (SFEMII) (Stem Cell Technologies catalog #09605); 100ng / ml hSCF (Peprotech); 100ng / ml hTPO (Peprotech); 100ng / ml hFlt- 3L (Peprotech); 100 ng / ml hIL-6 (Peprotech); StemRegenin1 (0.75 μM final concentration) Cayman Chemical
[0140] Freezing medium: Cryostor CS10
[0141] Cell isolation reagents: Human UCB CD34+ Enrichment Kit (Stem Cell Technologies; variations depending on source).
[0142] Other reagents: Neon kit (Thermo Fisher Scientific, various options depending on amount and desired tip size).
[0143] Thaw samples: Thaw cells in pre-warmed medium (37°C) using the same type of medium as used for cultivation. Add 1 mL of medium to a sterile 15 mL conical tube. Thaw frozen vials in a 37°C water bath until a single ice crystal remains. Immediately move the vial to a biosafety cabinet, spray with 70...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com