Method for preparing bis-(5-formylfurfuryl)ether from 5-hydroxymethylfurfural
A technology of formylfurfuryl and hydroxymethylfurfural, applied in chemical recovery, organic chemistry, etc., can solve the problems of poor hydrothermal stability, non-crystallization of pore walls, poor thermal stability, etc., and achieve easy recovery, maintain catalytic activity, strong The effect of water resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-7
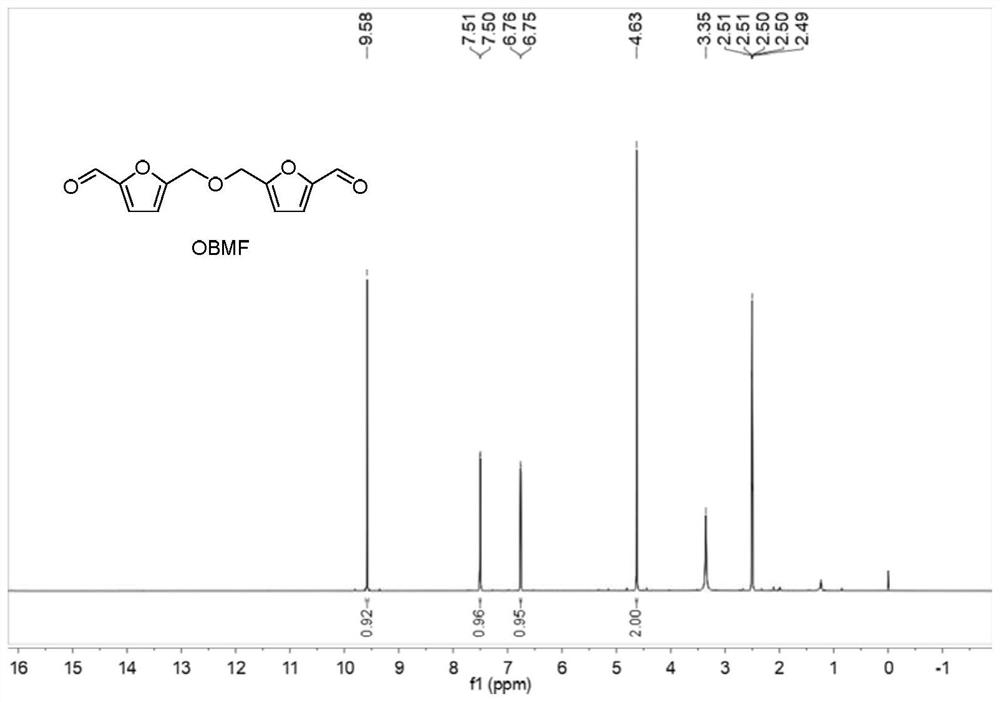
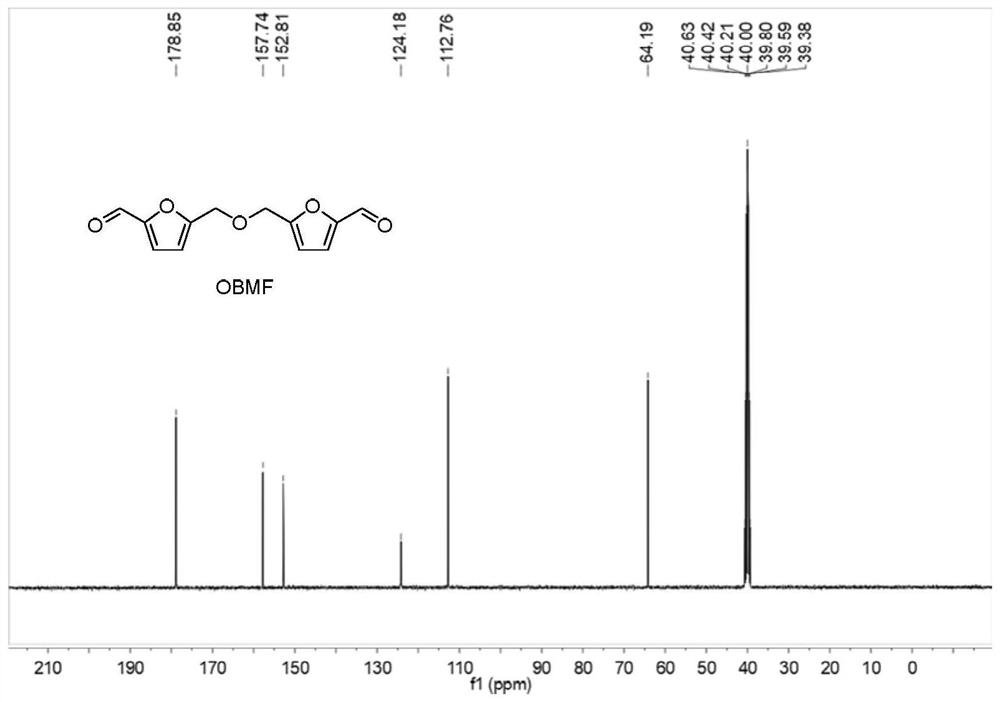
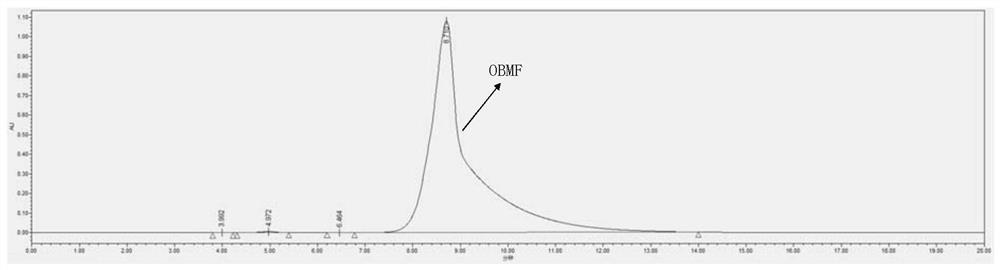
[0037] Add 250 mg of 5-hydroxymethylfurfural and 10 mL of toluene to a 25 mL reaction tube, heat to 90 °C under reflux, add 5 mg of catalyst (Zr(OTf) 4 、Hf(OTf) 4 , Nb(OTf) 5 、Ta(OTf) 5 , W(OTf) 6 、Sc(OTf) 3 and Yb(OTf) 3 ), and continued to stir for 6 hours. After the reaction, cool to room temperature, take samples, and send to HPLC for detection. The specific catalysts and test results are listed in Table 1, numbered 1-7. Preparation of bis-(5-formyl furfuryl) ether 1 H NMR diagram, 13 C NMR chart and HPLC chart as Figure 1-3 shown.
[0038] Table 1. The detection result of embodiment 1-7
[0039]
[0040]
Embodiment 8-11
[0042] Add 250 mg of 5-hydroxymethylfurfural and 10 mL of toluene to a 25 mL reaction tube, heat to a certain temperature under reflux, and add 5 mg of Yb(OTf) 3 , kept stirring for 6 hours. After the reaction, cool to room temperature, take samples, and send to HPLC for detection. The specific reaction temperature and detection results are listed in Table 2, and the serial numbers are 8-11.
[0043] Table 2. The detection result of embodiment 8-11
[0044]
Embodiment 12-15
[0046] Add 250mg of 5-hydroxymethylfurfural and 10mL of toluene to a 25mL reaction tube, heat to 110°C under reflux, add 5mg of Yb(OTf) 3 , keep stirring for a certain period of time. After the reaction, cool to room temperature, take samples, and send to HPLC for detection. The specific reaction time and detection results are listed in Table 3, and the serial numbers are 12-15.
[0047] Table 3. The detection result of embodiment 12-15
[0048]
[0049]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com