Treatment and prevention of house dust mite allergies
A technology for dust mites and allergens, applied in the directions of allergen antigen components, antibody medical components, chemical instruments and methods, etc., can solve the problems of HDM allergy patients who cannot be treated and cannot be induced.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0150] Example 1: Design of allergen fragments for fusion proteins of the invention
[0151] Peptides spanning the target allergen sequence were determined based on amino acid surface exposure predictions determined from the ProtScale bioinformatics tool of the ExPASY server (http: / / web.expasy.org / protscale / ). If the peptides do not contain a cysteine residue in their sequence, a cysteine is added to their N- or C-termini to allow them to be conjugated to keyhole limpet hemocyanin (KLH) . Peptides were synthesized using an Applied Biosystems Model 433A peptide synthesizer (Foster City, USA) and then purified by preparative high-performance liquid chromatography (HPLC) (Dionex, Thermofischer Scientific, USA) (Focke et al., FASEB J.2001; 15(11): 2042-4). The size and identity of the peptides were confirmed by mass spectrometry (Bruker, Austria).
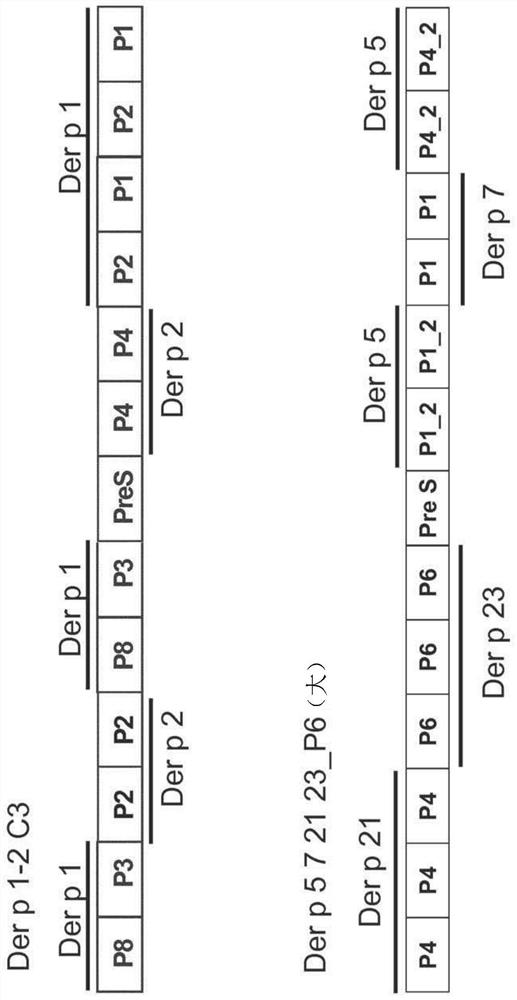
[0152] Table I lists allergen fragments derived from the house dust mite allergen Der p 1
[0153]
[0154]
[0155] ...
Embodiment 2
[0168] Embodiment 2: recombinant production of protein
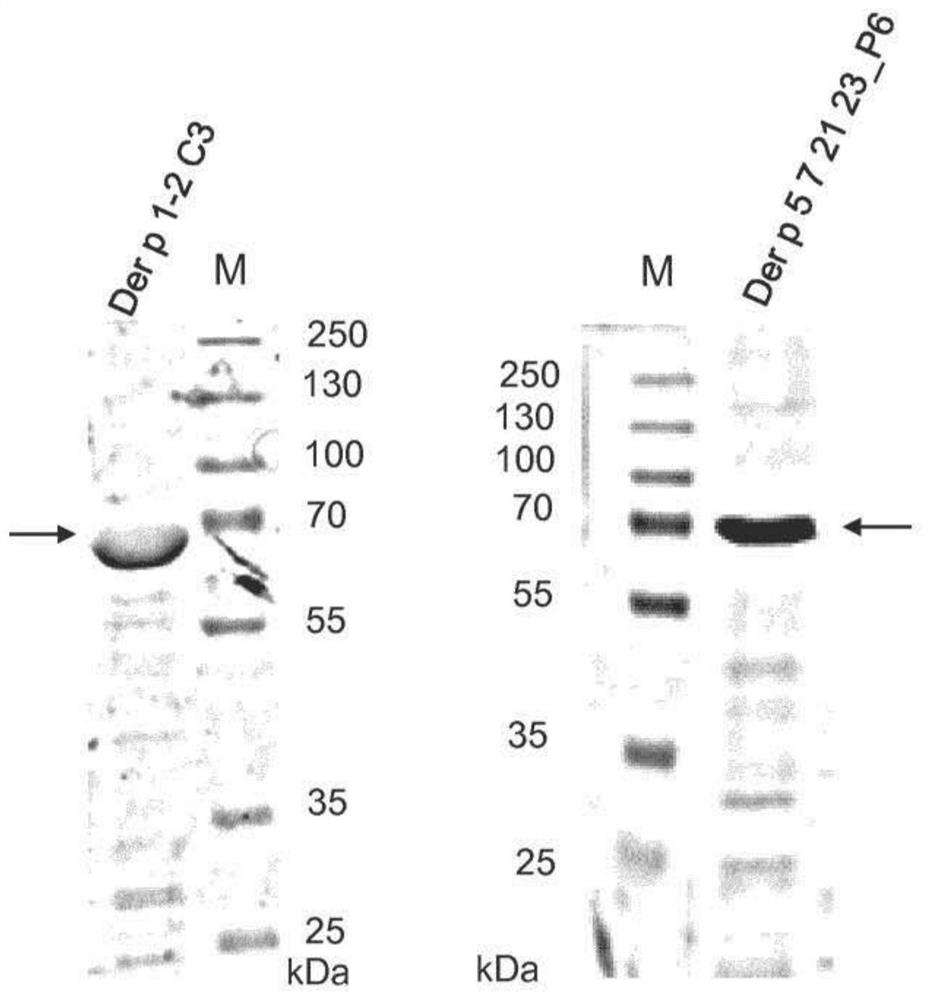
[0169] Genes encoding the fusion proteins Der p 1-2C3 and Der p 5 7 21 23_P6 (large) were synthesized (codon-optimized for E. coli expression) (ATG: Biosynthesis, Merzhausen, Germany and GenScript, Piscataway, USA), and It was inserted into the NdeI / XhoI site of pET-27b (Novagen, Germany). Recombinant proteins were expressed in E. coli strain BL21-Gold (DE3). Der p 1-2C3 with an OD600 of 0.4 was induced by adding 0.5 mM IPTG to the bacterial culture and incubating at 37°C for 3 hours (see figure 1 ; SEQ ID No.27) expression. Der p 5 7 21 23_P6 (large) with an OD600 of 0.6 was induced by adding 1 mM IPTG and incubating the culture at 37 °C for 2.5 h (see figure 1 ; SEQ ID No. 28).
[0170] After harvest and addition of protease inhibitors, an inclusion body preparation is performed to remove soluble bacterial proteins. The pellet containing partially purified Der p 1-2C3 protein was dissolved in 6M urea, 10 mM Tris...
Embodiment 3
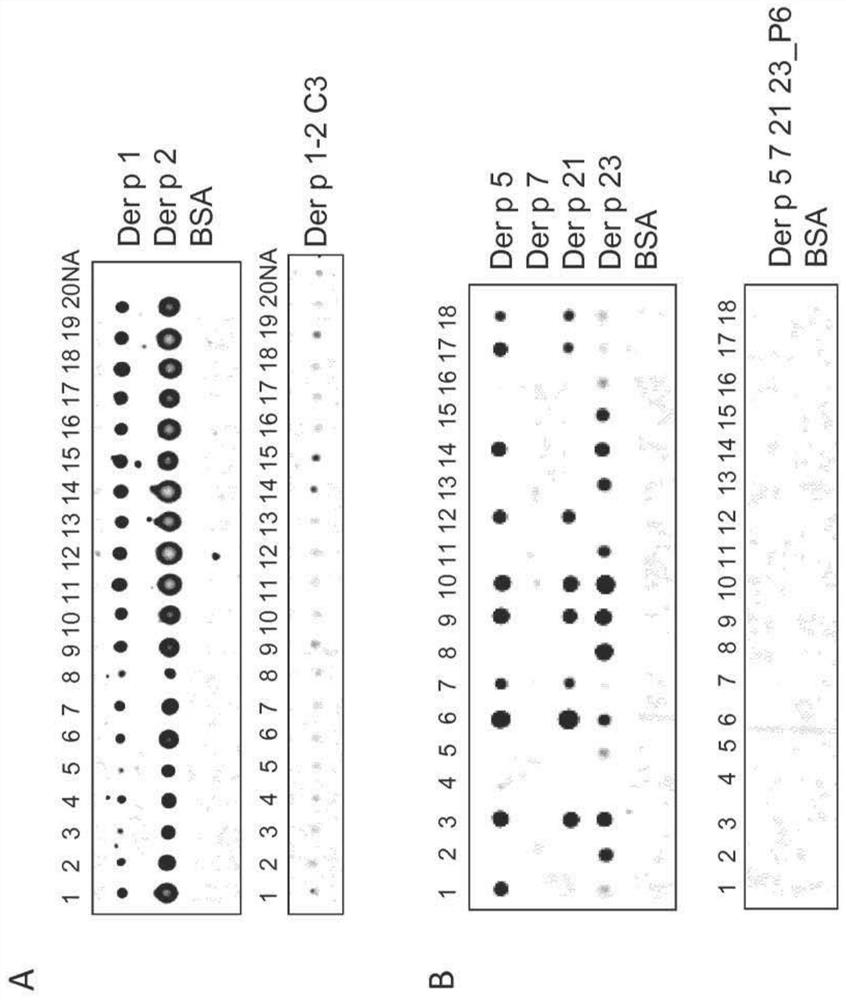
[0173] Example 3: IgE reactivity assay
[0174]The IgE reactivity of the construct of Example 2 was determined by immunoblotting (Curin M, et al., Sci Rep. 22(2017):12135). Contains 0.5 μg of purified nDer p 1 (natural isolate; accession number: PDB: 3RVW_A; reference method: Hales, B.J. et al., Clin Exp Allergy. 30(2000): 934–943), rDer p 2 (recombinantly produced Der p 2; sequence published in Chen KW et al., Allergy.67(2012): 609-21; reference method: Chen, K, et al., MolImmunol.45(2008): 2486–2498.), rDer p 5( GeneBank: X17699; Reference method: Weghofer M et al., Int Arch Allergy Immunol.147(2008):101-9.), rDer p 7 (GeneBank: U37044; Reference method: Resch Y et al., Clin Exp Allergy.41( 2011): 1468-77), rDer p 21 (GeneBank: DQ354124; reference method: Weghofer M et al., Allergy.63(2008): 758-67), rDer p 23 (GeneBank: EU414751.1; reference method: Weghofer Aliquots of M et al., J Immunol.190 (2013): 3059-67), Der p 1-2C3 (see Example 2), Der p 5 7 21 23_P6 (large) (se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com