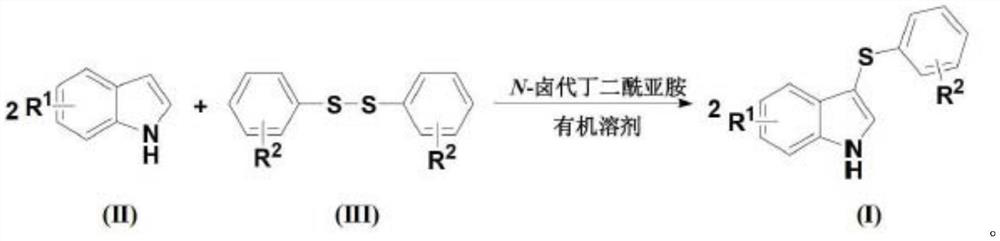
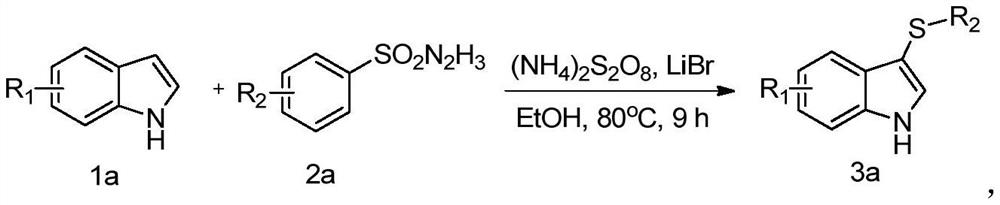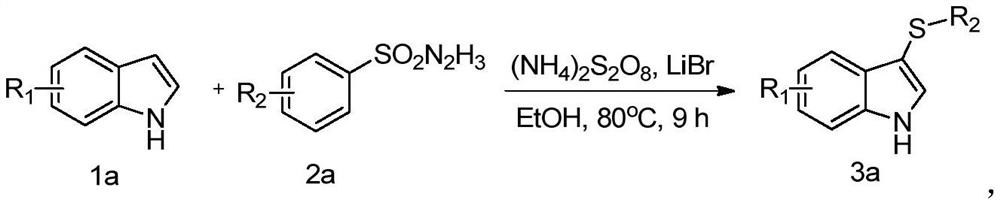Method for high-selectivity synthesis of 3-thio indole
A high-selectivity, indole technology, applied in organic chemistry and other directions, can solve the problems of poor selectivity, large amount of reagents, harsh reaction conditions, etc., and achieve the effects of reducing preparation cost, convenient operation and rapid reaction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A preparation method of 3-(4-methylphenylthio)indole / 3-(p-tolylthio)-1H-indole, comprising the following steps:
[0028] 1) Weigh 0.5mmol of indole, 0.6mmol of p-toluenesulfonyl hydrazide, 0.25mmol of lithium bromide, 0.1,,mmol of ammonium persulfate and add them into 2mL of ethanol solution and mix well, then stir and react at 80°C for 8-10h, to obtain a reaction mixture;
[0029] 2) The reaction mixture was dried and concentrated successively, and then using ethyl acetate / petroleum ether (1:30-1:10) as a developing solvent, the concentrate was separated by column chromatography to obtain 95.7 mg of the target product (yield is 80%).
[0030] Carry out NMR characterization to above-mentioned target product, the result is as follows:
[0031] 1 H-NMR (500MHz, CDCl 3 ):δ=8.37(s,1H), 7.67(d,J=7.9Hz,1H),7.46(d,J=11.5,5.3Hz,2H),7.30(t,J=13.0,5.1Hz,1H) ,7.21(t,J=7.5Hz,1H),7.09(d,J=8.1Hz,2H),7.02(d,J=8.1Hz,2H),2.30(s,3H);
[0032] 13 C-NMR (126MHz, CDCl 3 ): δ=136.5, ...
Embodiment 2
[0034] A preparation method of 3-(p-methoxyphenylthio)-1H-indole, comprising the following steps:
[0035] 1) Weigh 0.5mmol of indole, 0.6mmol of p-methoxybenzenesulfonyl hydrazide, lithium bromide, and ammonium persulfate and add them to 2mL of ethanol solution and mix well, then stir and react at 80°C for 8-10h to obtain a reaction mixture liquid;
[0036] 2) After the reaction mixture was dried and concentrated in sequence, the concentrate was separated by column chromatography using ethyl acetate / petroleum ether (1 / 30-1 / 10, v / v) as a developing solvent to obtain 107.2 mg of the target Product (84% yield).
[0037] Carry out NMR characterization to above-mentioned target product, the result is as follows:
[0038] 1 H-NMR (500MHz, CDCl 3 ):δ=8.42(s,1H),7.73(d,J=8.0Hz,1H),7.39(dd,J=12.6,8.5Hz,2H),7.30(t,J=11.1Hz,1H),7.23 (m,3H),6.78(d,J=8.8Hz,2H),3.77(s,3H);
[0039] 13 C-NMR (126MHz, CDCl 3 ): δ=157.8, 136.5, 130.1, 129.5, 129.0, 128.6, 123.0, 120.8, 119.6, 114.5, 1...
Embodiment 3
[0041] A preparation method of 3-(4-bromophenylthio) indole, comprising the following steps:
[0042]1) Weigh 0.5mmol of indole, 0.6mmol of brosenesulfonyl hydrazide, lithium bromide, and ammonium persulfate and add them into 2mL of ethanol solution and mix evenly, then stir and react at 80°C for 8-10h to obtain a reaction mixture;
[0043] 2) After the reaction mixture was dried and concentrated in sequence, the concentrate was separated by column chromatography using ethyl acetate / petroleum ether (1 / 30-1 / 10, v / v) as a developing solvent to obtain 115.5 mg of the target Product (76% yield).
[0044] Carry out NMR characterization to above-mentioned target product, the result is as follows:
[0045] 1 H-NMR (500MHz, CDCl 3 ):δ=8.47(s,1H),7.60(d,J=7.9Hz,1H),7.49(dd,J=16.6,4.8Hz,2H),7.30(dd,J=15.2,7.6Hz,3H) ,7.21(t,J=7.5Hz,1H),6.99(d,J=8.3Hz,2H);
[0046] 13 C-NMR (126MHz, CDCl 3 ): δ=138.6, 136.5, 131.7, 130.8, 128.8, 127.5, 123.3, 121.1, 119.5, 118.4, 111.7, 102.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com