Flexible MnNiTi-based magnetic phase change alloy material as well as preparation method, regulation and control method and application thereof
A technology of alloy material and magnetic phase transition, applied in magnetic materials, refrigeration and liquefaction, and magnetism of inorganic materials, etc., can solve the problems of poor practical feasibility and uncontrollable magnetic change, and achieve large-rotation magnetocaloric effect, magnetization and magnetic The effect of thermal effect enhancement and large magnetic entropy change effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
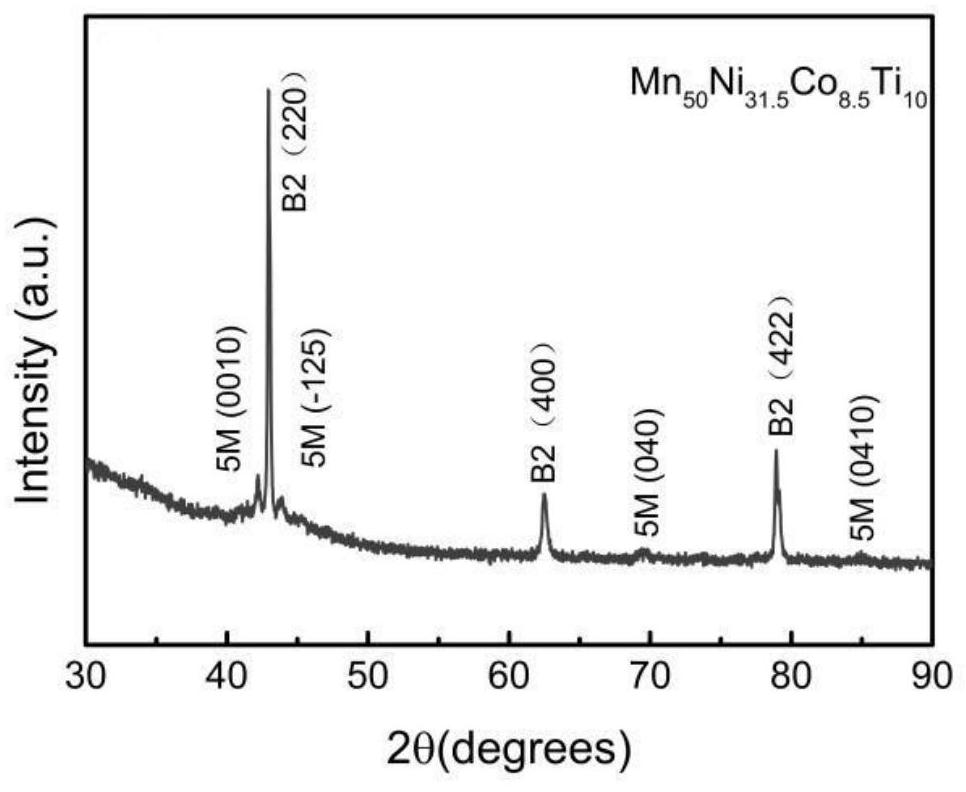
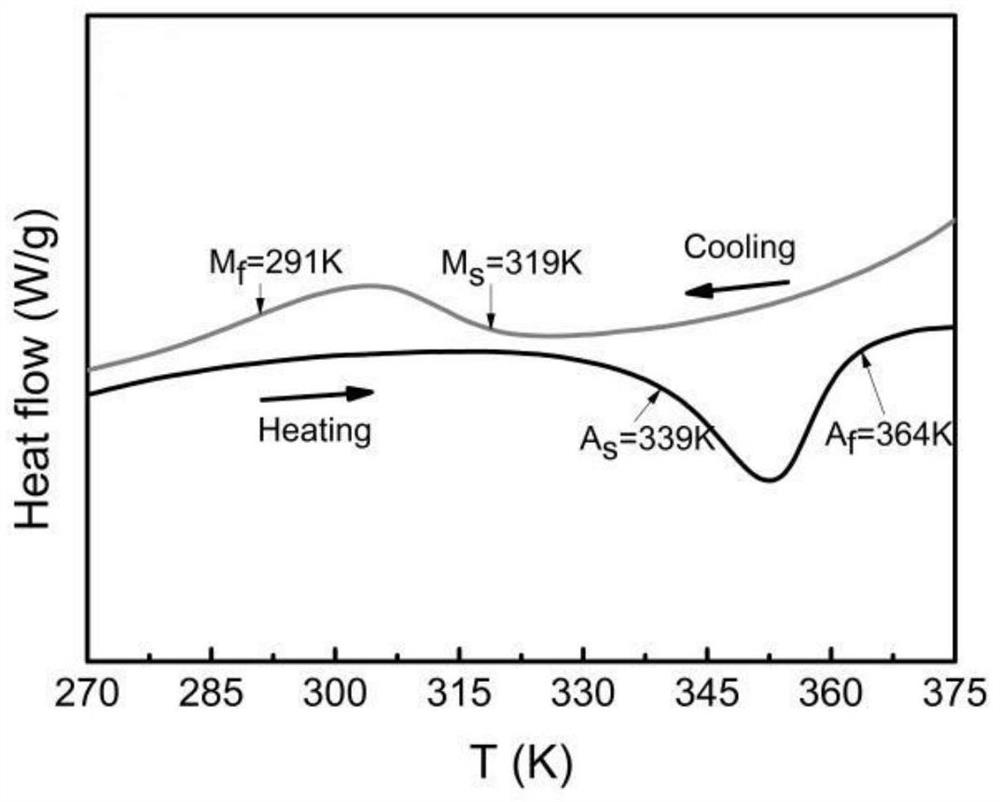
[0058] (1) Ingredients: According to the chemical formula Mn 50 Ni 31.5 co 8.5 Ti 10 The ratio in the formula is to weigh high-purity raw materials Ni, Mn, Co, Ti, and carefully polish off the oxide layer on the surface of the required transition metal elements before mixing.
[0059] Taking Mn element as an example, it needs to be cleaned and smelted before ingredients to ensure the purity of raw materials. The specific steps are as follows:
[0060] 1) Put a certain amount of Mn elemental substance in the beaker, and then pour a dilute hydrochloric acid solution diluted with water with a volume ratio of about 1:1 to make a chemical reaction, and stir it quickly with a glass rod during the reaction;
[0061] 2) When the oxide disappears and the Mn surface shows a bright metallic luster, quickly pour out the waste solution after the reaction in the beaker:
[0062] 3) Rinse the reacted metal Mn with deionized water, rinse twice and then rinse twice with industrial alcohol;...
Embodiment 2
[0077] The difference between embodiment 2 and embodiment 1 is that the chemical formula of the flexible MnNiTi-based magnetic phase change alloy material (phase change ribbon material) is: Mn 50 Ni 28 co 10 Ti 12 , step (4) is different, and the rest of the process is exactly the same.
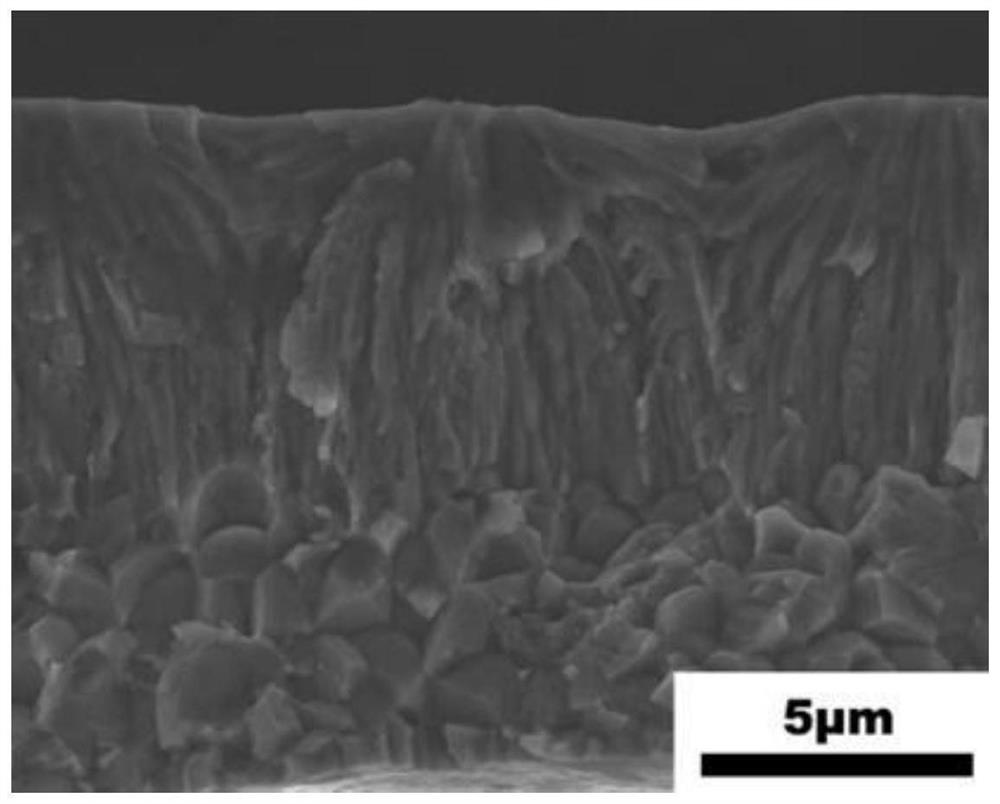
[0078] Step (4): Fully dissolve polyethylene glycol (PEG) and water at a mass ratio of 1:7; magnetically stir the prepared solution at a heating temperature of 50°C for 3 hours to obtain a PEG sol; Mn 50 Ni 28 co 10 Ti 12 Thin strips were placed on glass slides, and were spin-coated with PVA for 90s by a homogenizer. During the spin coating process, the speed of the homogenizer was 1000 r / min, so that Mn 50 Ni 28 co 10 Ti 12 The thin tape was completely adhered to the upper surface of the glass slide; the composite thin tape obtained after spin coating was dried at a heating temperature of 60°C and the drying time was 5 min to obtain a composite thin tape; then polydimethyl The sil...
Embodiment 3
[0080] The difference between embodiment 3 and embodiment 1 is that the chemical formula of the flexible MnNiTi-based magnetic phase change alloy material is: Mn 50 Ni 33 co 8 Ti 9 , step (4) is different, and the rest of the process is exactly the same.
[0081] Step (4): Fully dissolve polyethylene glycol (PEG) and water at a mass ratio of 1:8; magnetically stir the prepared solution at a heating temperature of 60°C for 2.5 hours to obtain a PEG sol; Mn 50 Ni 33 co 8 Ti 9 Thin strips were placed on glass slides, and were spin-coated with PVA for 90s by a homogenizer. During the spin coating process, the speed of the homogenizer was 1000 r / min, so that Mn 50 Ni 33 co 8 Ti 9 The thin tape was completely adhered to the upper surface of the glass slide; the composite thin tape obtained after spin coating was dried at a heating temperature of 70°C and the drying time was 3 min to obtain a composite thin tape; then the polyethylene naphthalate The glycol ester (PEN) sub...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com