1,2,5-polysubstituted imidazole derivative as well as synthesis method and application thereof
A synthesis method and imidazole technology are applied in the field of 1,2,5-polysubstituted imidazole derivatives and their synthesis, which can solve the problems of insufficient substrate use range, poor regioselectivity, and difficulty in obtaining raw materials, and achieve high atomic The effect of economy, low catalyst dosage and wide substrate versatility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
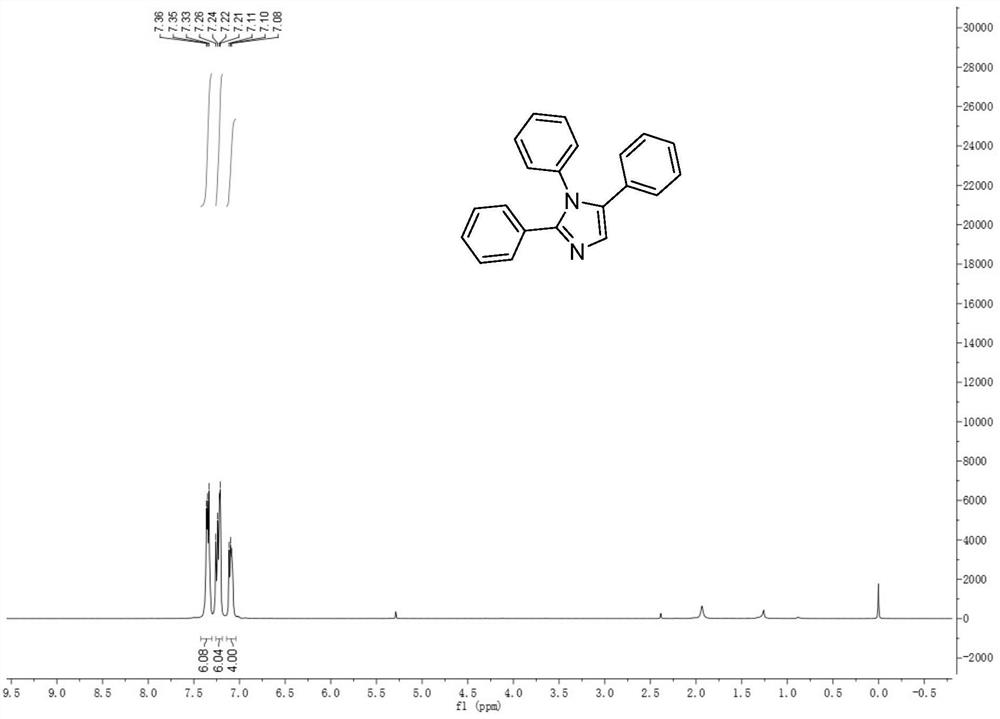
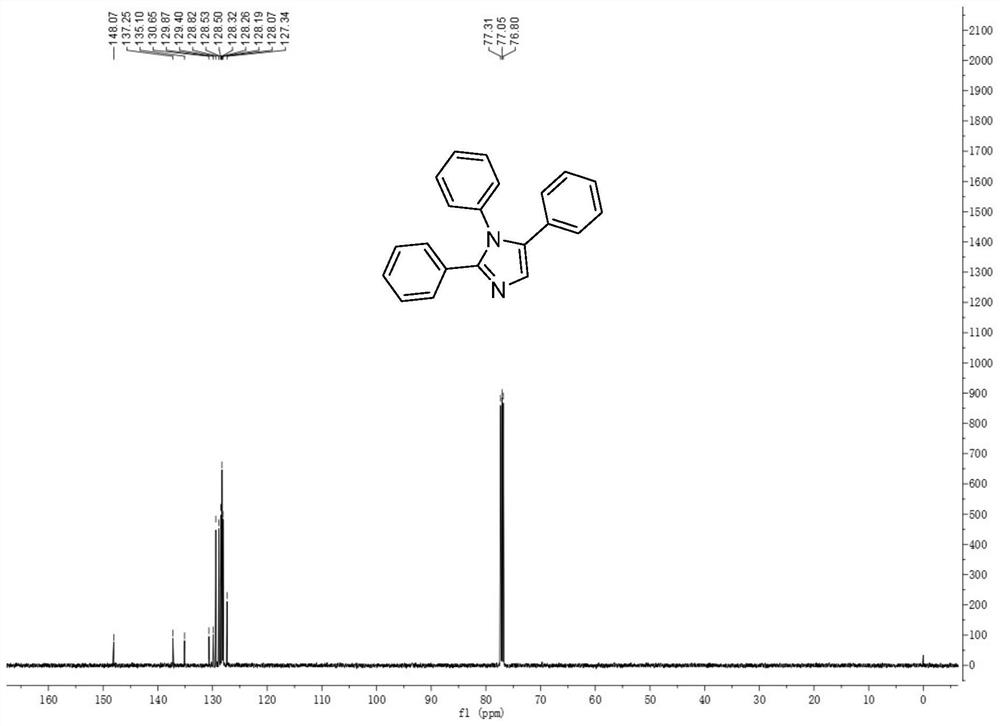
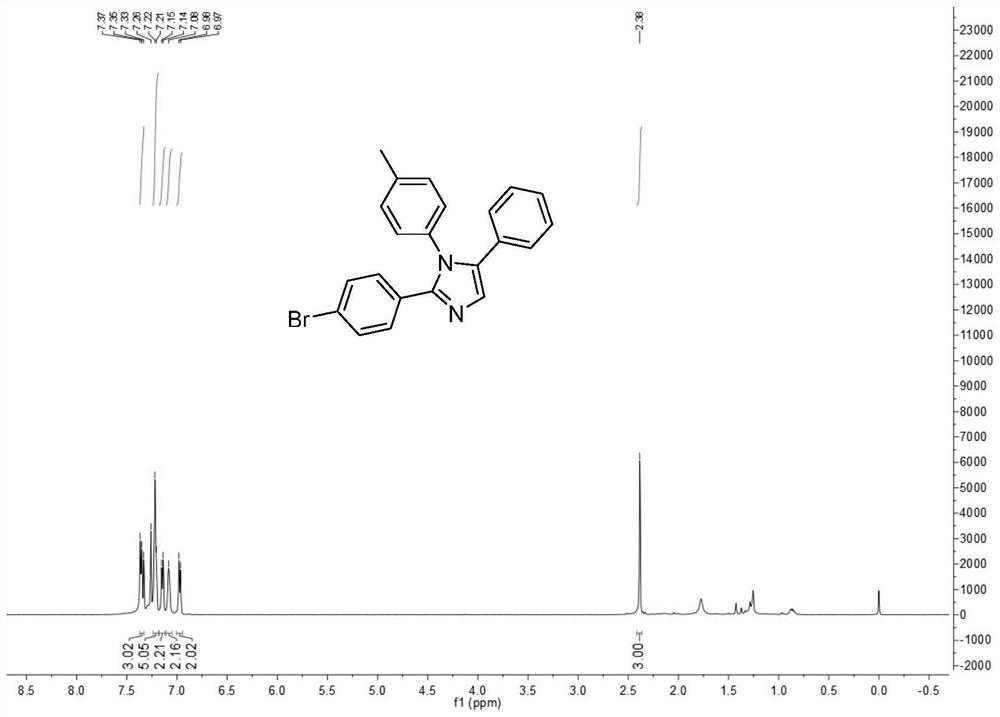
[0032] The structure of the 1,2,5-multi-substituted imidazole derivatives of the present invention is shown in formula (1):
[0033] (1)
[0034] Among them, R 1 is phenyl, C 1 -C 10 Alkyl-substituted phenyl, halogen-substituted phenyl, C 1 -C 10 Alkoxy-substituted phenyl, naphthyl, furyl, thienyl; R 2 is phenyl, C 1 -C 10 Alkyl-substituted phenyl, halogen-substituted phenyl, C 1 -C 10 Alkoxy-substituted phenyl, naphthyl, pyrrolyl, furyl, thienyl; R 3 is phenyl, C 1 -C 10 Alkyl-substituted phenyl, halogen-substituted phenyl, C 1 -C 10 Alkoxy substituted phenyl, naphthyl, thienyl.
[0035] Preferably, the R 1p-methylphenyl, p-chlorophenyl, p-bromophenyl, p-methoxyphenyl, 3,5-dichlorophenyl, 3,5-dimethylphenyl, m-bromophenyl, m-methylphenyl Oxyphenyl, o-methoxyphenyl, o-benzyloxyphenyl, o-fluorophenyl, 2-naphthyl, 2-pyrrolyl, 2-furyl, 2-thienyl; R 2 p-methylphenyl, p-chlorophenyl, p-bromophenyl, p-methoxyphenyl, 3,5-dichlorophenyl, 3,5-dimethylphenyl, m-bromo...
Embodiment 2
[0037] The synthesis method of 1,2,5-multi-substituted imidazole derivatives of the present invention uses styryl azide, aromatic aldehyde, and aromatic amine as raw materials, uses rhodium acetate and phosphoric acid as catalysts, and uses organic solvents as solvents to undergo a one-step reaction , to obtain the 1,2,5-multi-substituted imidazole derivatives; the reaction process is shown in the reaction formula (I):
[0038] (I)
[0039] Put the styryl azide, aromatic aldehyde, aromatic amine, rhodium acetate, and phosphoric acid in a reaction flask, then add an organic solvent to form a mixed solution, and react by heating to obtain the 1,2,5-multi-substituted imidazole kind of derivatives.
[0040] The molar ratio of the raw materials is styryl azide compound: aromatic aldehyde: aromatic amine: rhodium acetate: phosphoric acid = (2.0-3.0): (1.0-1.5): (1.0-1.5): (0.02-0.05): ( 0.1-0.2).
[0041] The organic solvent is 1,2-dichloroethane or aromatic hydrocarbon; wherei...
Embodiment 3
[0045] The synthesis method of the 1,2,5-multi-substituted imidazole derivatives of this embodiment is different from that of Example 2 in that the molar ratio of the raw materials is styryl azide compound: aromatic aldehyde: aromatic amine: rhodium acetate : phosphoric acid=2.0:1.0:1.0:0.02:0.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com