Lentivirus packaging vector system, lentivirus, construction method of lentivirus and kit
A technology of lentiviral packaging and vector system, applied in the field of lentiviral packaging vector system, can solve the problems of limiting the efficiency of SARS-CoV-2 drug development and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
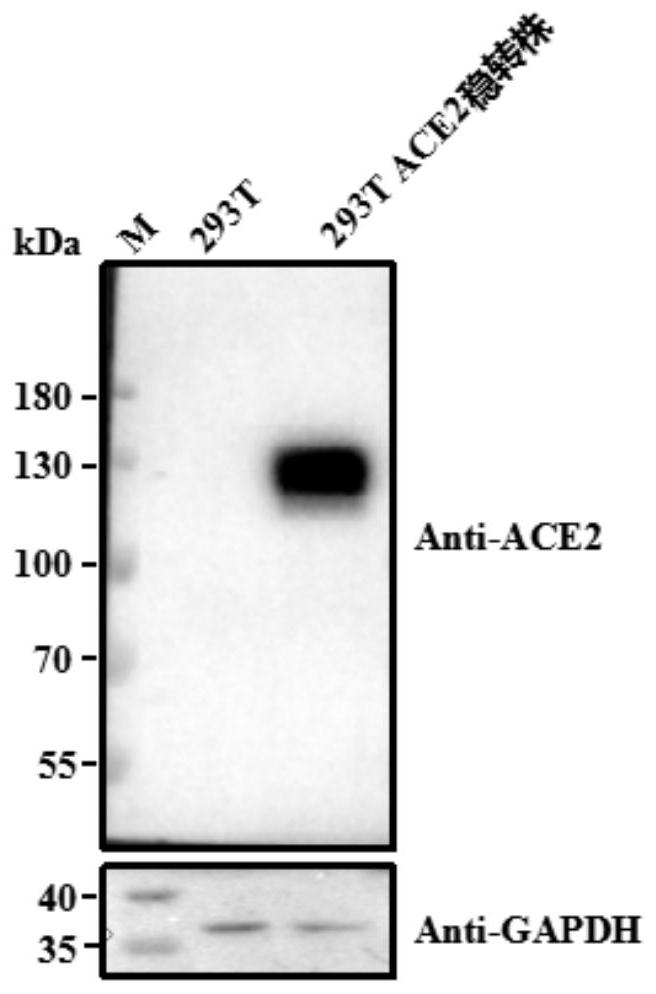
[0042] Preparation of cell lines expressing ACE2 receptor
[0043] (1) Pack pLV[Exp]-Bsd-CMV>hACE2 (from Yunzhou Bio) into a lentivirus with VSV-G;
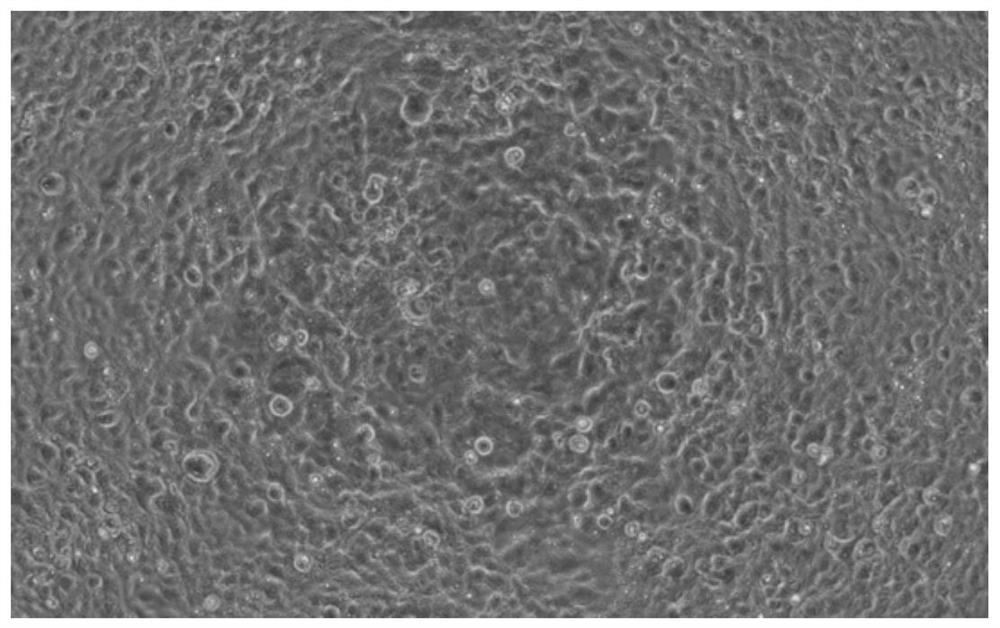
[0044] (2) Transduce the HKE293T cells with the lentivirus in step (1), screen with a suitable concentration of blasticidin Bsd (Blasticidin) until the blank cells die, and obtain a stable transfection strain expressing the ACE2 receptor, which is denoted as HEK293T- ACE2 stable transfection strain. The image of HEK293T-ACE2 stable transfection strain under 200× microscope is as follows: figure 1 shown. Depend on figure 1 It can be seen that HEK293T-ACE2 stable transfection plants are in good growth state.
[0045] (3) Extract the genomic DNA of the HEK293T-ACE2 stable transfection strain and perform qRT-PCR detection, wherein the qPCR primers are as follows: ACE2-F: 5'-GCAGCCCACCTAAGCATT-3' (SEQ ID No: 6); ACE2-R: 5' - CCATCCACCTCCACTTCTCT-3' (SEQ ID No: 7); HKE293T was used as blank control.
[0046] The qRT-PCR results s...
Embodiment 2
[0050] Detect the expression level of S protein after transiently transfecting HEK293T cells with different S protein expression vectors (the vector is used as an envelope protein expression plasmid during the virus packaging process)
[0051] (1) Transient HEK293T cells with the same amount of 10 kinds of S protein expression vectors, each using Lipofectamine 2000 as a transfection reagent, to obtain HEK293T cells corresponding to each S protein expression vector. Among the 10 S protein expression vectors, the nucleotide sequence of S_a is shown in SEQ ID No: 8, which encodes the wild-type S protein; the nucleotide sequence of S_c is shown in SEQ ID No: 9, which encodes the D614G mutant S Protein; the nucleotide sequence of S_b is as shown in SEQ ID No: 10, is optimized on the basis of S_a and adds the nucleic acid fragment corresponding to the purification tag 3×Flag (that is, the nucleotide sequence is as shown in SEQ ID No: 3 The nucleic acid fragment corresponding to the ...
Embodiment 3
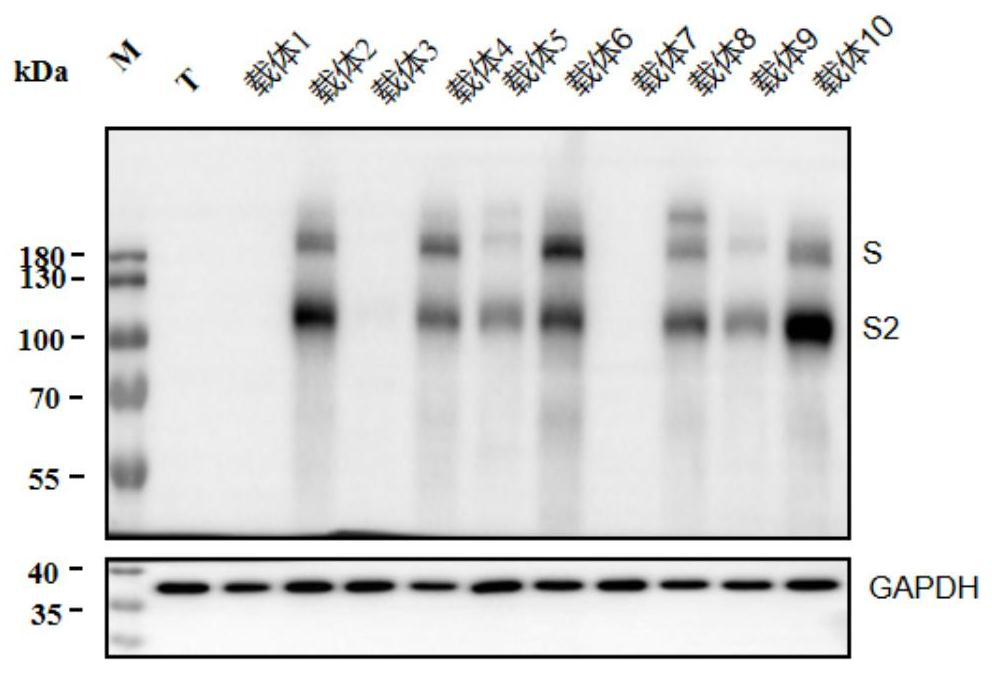
[0058] Comparison of lentiviral packaging effects of different S protein expression vectors
[0059] The partial S protein expression vector of embodiment 2 is selected to carry out the lentiviral packaging test, and the materials used in the test include the following:
[0060] Vector 1: pRP[Exp]-CMV>S_a; Vector 2: pRP[Exp]-CMV>S_b; Vector 4: pRP[Exp]-CMV-human beta globin intron>S_b; Vector 6: pRP[Exp]-CAG >S_b; Vector 7: pRP[Exp]-CMV-humanbeta globin intron>S_c; Vector 8: pRP[Exp]-CMV-human beta globin intron>S_d; Vector 9: pRP[Exp]-CAG>S_c; Vector 10 : pRP[Exp]-CAG>S_d; lentiviral packaging expression plasmid: pLV[Exp]-CMV>EGFP; gag-pol expression plasmid (hereinafter referred to as PLV4): gag gene and pol gene containing lentivirus; rev expression plasmid ( Hereinafter referred to as PLV5): encoding lentiviral Rev regulatory factor; RIPA lysate (strong), Biyuntian P0013B; Sars SpikeProtein Antibody (SARS-S protein antibody), (novusbio NB100-56578SS); horseradish peroxida...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com