Serum-free high-density fermentation culture process for serum type 9 streptococcus suis
A high-density fermentation and Streptococcus suis technology, applied in the field of serum-free high-density fermentation and culture technology of serum type 9 Streptococcus suis, can solve the problems of lack of production and culture technology and inconvenience of serum type 9 Streptococcus suis, and achieve the prevention of serum type 9 Streptococcus suis Streptococcus suis, simple formula, fast bacterial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: 40L primary fermentation tank fermentation
[0036] Strain: SQ strain of Streptococcus suis serotype 9.
[0037] Seed culture: wash TSA (5% sheep blood) agar slant with 2ml TSB liquid medium, inoculate in TSB (5% adult bovine serum) liquid medium, culture at 37°C with shaking for 3.5h, OD 600nm The value reaches 0.4~0.8.
[0038] Fermentation culture: the fermentation medium is adjusted to pH 7.2 with ammonia water, sterilized at 121°C for 30 minutes, inoculated with 2% (V / V) inoculum in the fermenter, the stirring speed is controlled at 150rpm, the dissolved oxygen is controlled to 30%, and the tank body is kept positive. When the pressure is 0.04Mpa and the OD600nm of the fermented bacteria liquid is 1.0, stop the aeration culture. Add ammonia water to control the pH at 7.2. Add 400 ml each of 1% glucose solution and 1% skim milk solution when the concentration of the fermentation broth reaches OD600nm=0.8, 1.5, and 2.0, and the flow rate is controlle...
Embodiment 2
[0039] Embodiment 2: 500L secondary tank fermentation
[0040] Strain: SQ strain of Streptococcus suis serotype 9.
[0041] Primary seed culture: wash TSA (5% sheep blood) agar slant with 2ml TSB liquid medium, inoculate in TSB (5% adult bovine serum) liquid medium, culture at 37°C for 3.5 hours on a shaking table, OD 600nm The value reaches 0.6.
[0042]Secondary seed culture: the fermentation medium is adjusted to pH 7.2 with ammonia water, sterilized at 121°C for 30 minutes, inoculated with 2% (V / V) inoculum in the fermenter, the stirring speed is controlled at 100rpm, the dissolved oxygen is controlled to 45%, and the tank is maintained The positive pressure of the body is 0.05Mpa, and the pH is controlled at 7.2 by feeding ammonia water. When the culture OD600nm reaches 0.8, it is transplanted into a 500L fermenter.
[0043] Fermentation culture: the fermentation medium is adjusted to pH 7.4 with NaOH, sterilized at 121°C for 30 minutes, inoculated with 2% (V / V) inoculu...
Embodiment 3
[0044] Example 3: Immunogenicity assay
[0045] Immunogenicity in 28-35 day old piglets:
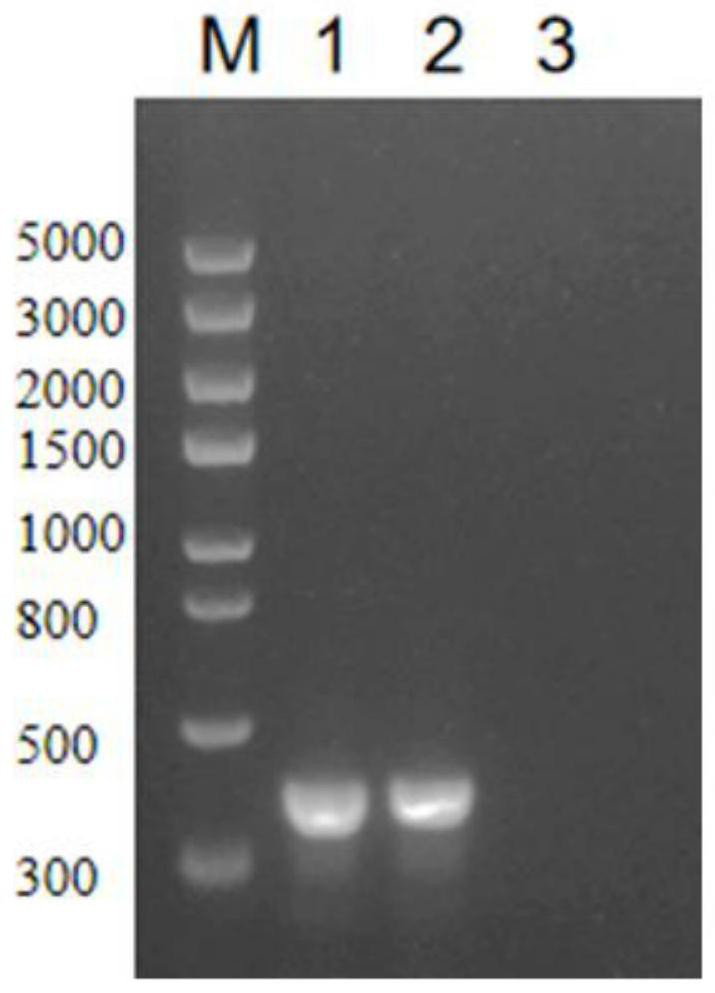
[0046] High-density fermented antigen prepared inactivated oil emulsion vaccine, 2ml / head, neck muscle immunization 5 healthy piglets aged 28-35 days, 28 days after immunization, together with 5 piglets of the control group, a minimum lethal dose (4.5 ×10 9 CFU / head) of the SQ strain viable bacterial liquid, observed 14 days after the challenge, and recorded the death of piglets, such as Figure 4 shown.
[0047] Immunogenicity on 18~22g clean grade Kunming mice
[0048] High-density fermented antigen prepared inactivated oil emulsion vaccine, 0.5ml / only, intraperitoneally immunized 10 Kunming rats of 18-22g clean grade, 21 days after immunization, together with 10 mice of the control group, intraperitoneally injected a minimum lethal dose (2.1×10 9 CFU / only) of the SQ strain live bacteria liquid, observed 7 days after the challenge, and recorded the death of the mice, such as Figu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com