Pharmaceutical composition for treating pulmonary tuberculosis and preparation method thereof
A composition and a technology for pulmonary tuberculosis, which are applied in the field of medicine, can solve the problems of safety, large reduction in drug content, unstable drug quality, etc., and achieve the effects of convenient use and storage, simple preparation, and improved drug efficacy and safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
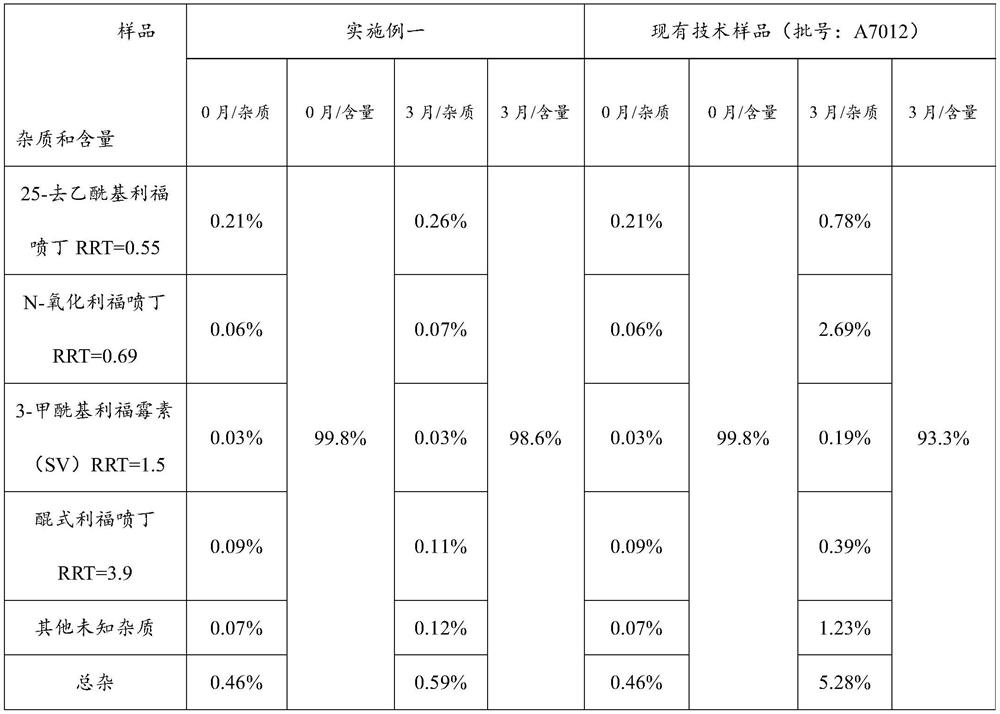
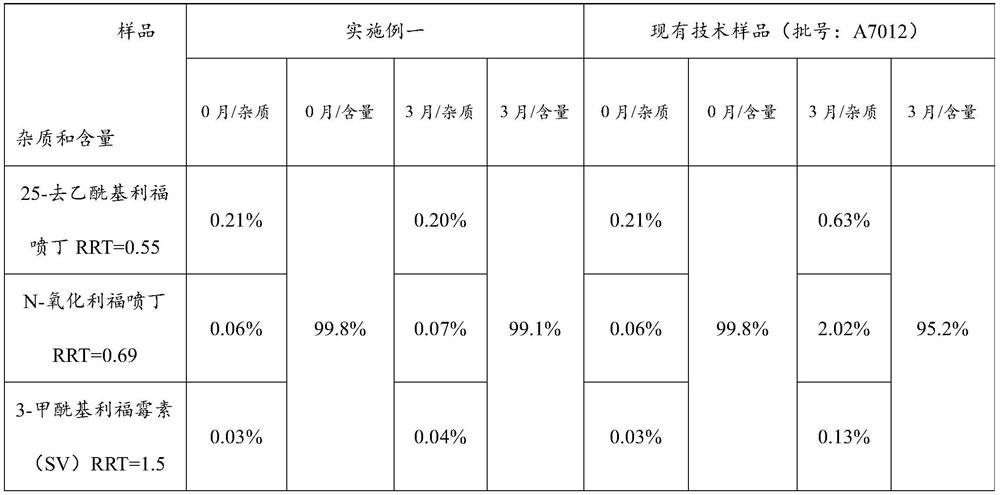
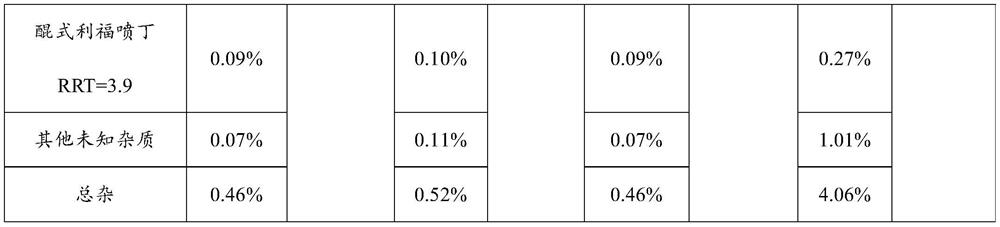
[0046] This embodiment discloses a new drug technology for pulmonary tuberculosis, including the following components and dosage:
[0047] Element Dosage / tablet Rifapentine 150g Carmine 1.03g ethanol 480g Croscarmellose Sodium 10g starch 25g Hypromellose 10g Sodium dodecyl sulfate 2.6g Calcium stearate 1.6g Polyacrylic resin (Eudragit EPO) 21g polyethylene glycol 4000 5g production 1000 pieces
[0048] Preparation Process:
[0049] Step 1: dissolving carmine in 80g of ethanol; adding polyacrylic acid resin into 400g of ethanol, stirring to dissolve, then adding polyethylene glycol 4000 and stirring to dissolve;
[0050] Step 2: Add the prescribed amount of rifapentine raw materials into the fluidized granulator and set the air intake volume to 12m 3 / h, the inlet air temperature is 45°C, the flow rate is 10rpm, and the atomization pressure is 0.04MPa. Spray the carmine solution into the raw mat...
Embodiment 2
[0055] This embodiment discloses a new drug technology for pulmonary tuberculosis, including the following components and dosage:
[0056] Element Dosage / tablet rifampicin 150g Carmine 1.03g ethanol 480g Croscarmellose Sodium 10g starch 25g Hypromellose 10g Sodium dodecyl sulfate 2.6g Calcium stearate 1.6g Ethyl cellulose 18.8g polyethylene glycol 4000 5g production 1000 pieces
[0057] Preparation Process:
[0058]Step 1: Dissolve carmine in 80g ethanol; add ethyl cellulose into 400g ethanol, stir to dissolve, then add polyethylene glycol 4000 and stir to dissolve;
[0059] Step 2: Add the prescribed amount of rifampin raw material into the fluidized granulator and set the air intake volume to 12m 3 / h, the inlet air temperature is 45°C, the flow rate is 10rpm, and the atomization pressure is 0.04MPa. Spray the carmine solution into the raw material;
[0060] Step 3: Continue to set the air i...
Embodiment 3
[0064] This embodiment discloses a new drug technology for pulmonary tuberculosis, including the following components and dosage:
[0065] Element Dosage / tablet Rifapentine 150g Amaranth 1.2g ethanol 480g Croscarmellose Sodium 10g starch 25g Hypromellose 10g Sodium dodecyl sulfate 2.6g Calcium stearate 1.6g Polyacrylic resin (Eudragit EPO) 21g polyethylene glycol 4000 5g production 1000 pieces
[0066] Preparation Process:
[0067] Step 1: dissolving carmine in 80g of ethanol; adding polyacrylic acid resin into 400g of ethanol, stirring to dissolve, then adding polyethylene glycol 4000 and stirring to dissolve;
[0068] Step 2: Add the prescribed amount of rifapentine raw materials into the fluidized granulator and set the air intake volume to 12m 3 / h, the inlet air temperature is 45°C, the flow rate is 10rpm, and the atomization pressure is 0.04MPa. Spray the carmine solution into the raw mat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com