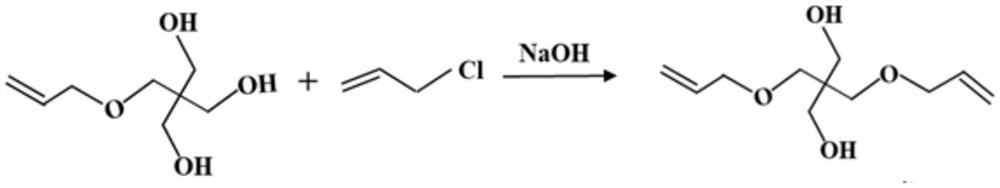
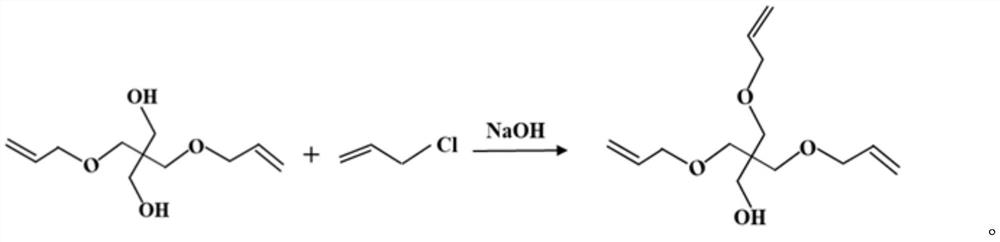
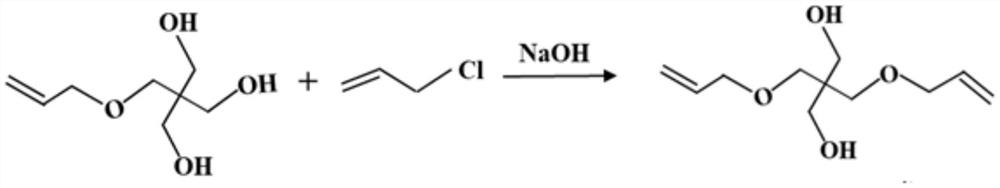
Preparation method of pentaerythritol triallyl ether
A technology of pentaerythritol triallyl ether and allyl chloride, which is applied in the field of preparation of pentaerythritol triallyl ether, can solve the problems of many steps in the production process, insufficient contact, residue, etc., and achieve good end-capping and post-treatment , by-product reduction, feed reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Put 352.0g of pentaerythritol monoallyl ether and 80.0g of sodium hydroxide into the reaction kettle, degas and dehydrate at 90°C for 3 hours, then add 153.0g of allyl chloride dropwise, and slowly add allyl chloride at 90°C Base chlorine, the reaction time of dropwise addition is 2 hours, after the end, keep warm for 3 hours; cool down to below 35°C, convert it into sodium chloride according to the molar amount of sodium hydroxide, add water to form a saturated sodium chloride solution, and let it stand for separation Layer for 1 hour, remove the lower layer aqueous solution, leave the upper semi-finished product to continue the follow-up reaction; add 84.0g sodium hydroxide to the semi-finished product, at 90 ° C, degassing, dehydration reaction time is 3 hours, then dropwise add 160.7g allyl chloride, Slowly add allyl chloride at 90°C, and the dropwise reaction time is 2 hours. After the end, keep warm for 3 hours; cool down to below 35°C, then add water according to ...
Embodiment 2
[0028] Put 352.0g of pentaerythritol monoallyl ether and 80.0g of sodium hydroxide into the reaction kettle, degas and dehydrate at 120°C for 7 hours, then add 153.0g of allyl chloride dropwise, and slowly add allyl chloride at 120°C Base chlorine, the dropwise reaction time is 4 hours. After the end, the heat preservation reaction is 6 hours; the temperature is lowered to below 35 ° C, converted into sodium chloride according to the molar amount of sodium hydroxide, and water is added to form a saturated sodium chloride solution. Layer for 3 hours, remove the lower layer of aqueous solution, leave the upper semi-finished product to continue the follow-up reaction; add 84.0g of sodium hydroxide to the semi-finished product, at 120 ° C, degassing, dehydration reaction time is 7 hours, then dropwise add 160.7g of allyl chloride, Slowly add allyl chloride at 120°C, and the dropwise reaction time is 4 hours. After the end, keep warm for 6 hours; cool down to below 35°C, then add wa...
Embodiment 3
[0031] Put 352.0g of pentaerythritol monoallyl ether and 80.0g of sodium hydroxide into the reaction kettle, degas and dehydrate at 120°C for 7 hours, then add 153.0g of allyl chloride dropwise, and slowly add allyl chloride at 120°C Base chlorine, the dropwise reaction time is 4 hours. After the end, the heat preservation reaction is 6 hours; the temperature is lowered to below 35 ° C, converted into sodium chloride according to the molar amount of sodium hydroxide, and water is added to form a saturated sodium chloride solution. Layer, remove the lower aqueous solution, leave the semi-finished product in the upper layer to continue the follow-up reaction; add 84.0g sodium hydroxide to the semi-finished product, at 90 ° C, degassing, dehydration The reaction time is 7 hours, then dropwise add 160.7g allyl chloride, at 90 Slowly add allyl chloride at ℃, and the dropwise reaction time is 2 hours. After the end, keep warm for 3 hours; cool down to below 35℃, then add water accord...
PUM
| Property | Measurement | Unit |
|---|---|---|
| color | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com