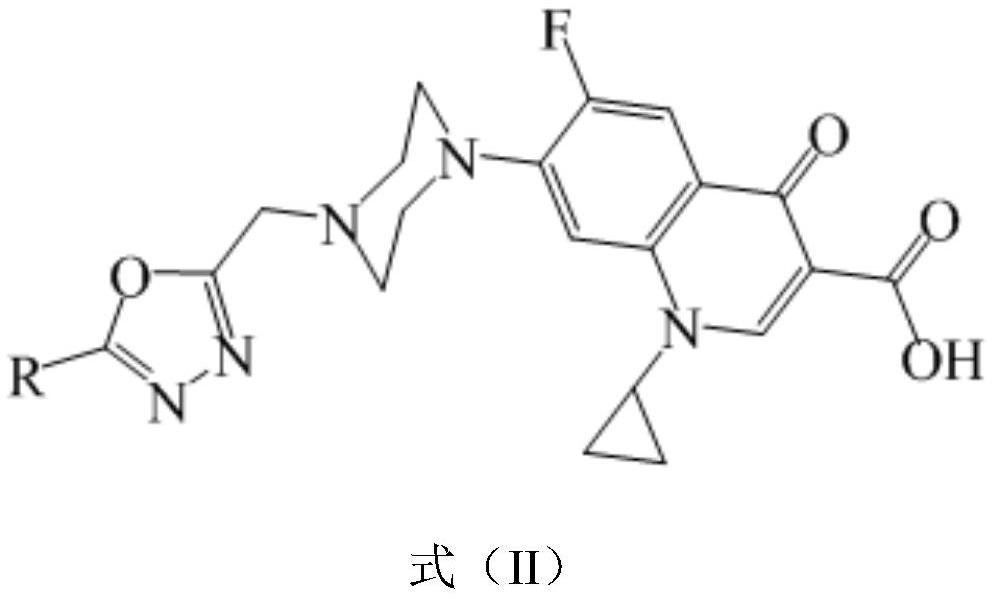
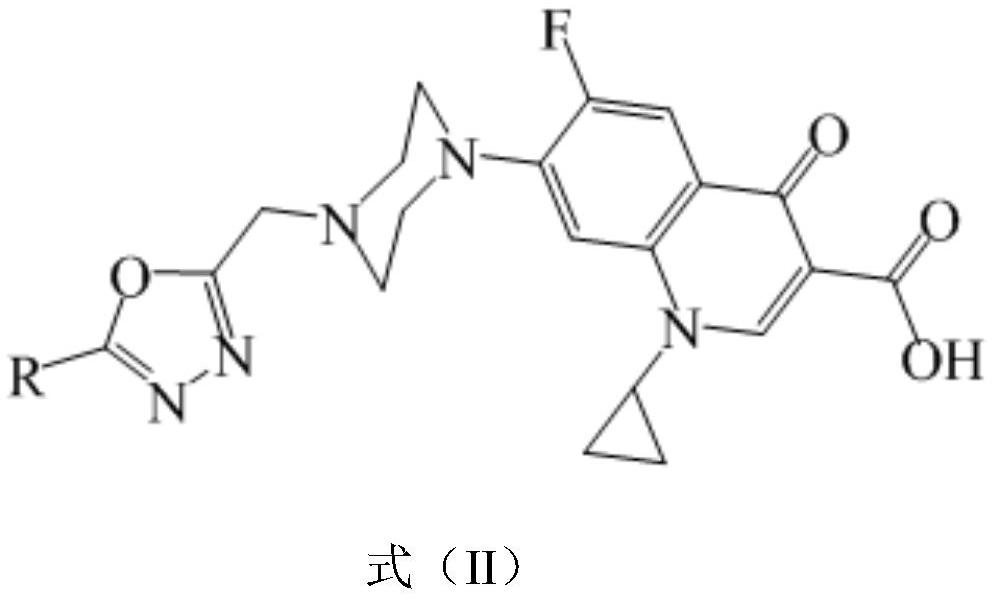
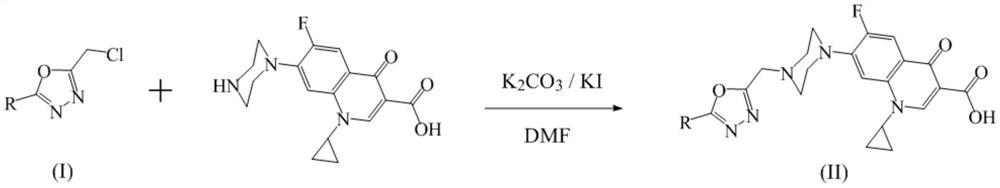
1, 3, 4-oxadiazole-ciprofloxacin heterozygote as well as preparation method and application thereof
A technology of ciprofloxacin and hybrid, which is applied in the field of antibacterial and can solve the problems of increased bacterial resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1-Cyclopropyl-6-fluoro-7-(4-((5-(furan-2-yl)-1,3,4-oxadiazol-2-yl)methyl)piperazin-1-yl )-4-oxy-1,4-dihydroquinoline-3-carboxylic acid (IIa) preparation steps:
[0028]
[0029] 8.0 mmol of furan-2-carboxyhydrazide, 9.6 mmol of chloroacetyl chloride and 30 mL of ethyl acetate were added to a 100 mL flask, and the reaction was stirred at room temperature for 3 hours. After the reaction was completed, the mixture was filtered and air-dried to obtain the precursor compound. 4.8 mmol of the precursor compound, 9.4 mmol of phosphorus oxychloride, and 20 mL of acetonitrile were added to a 100 mL flask, and the reaction was stirred at 70 ° C for 20 hours. After the reaction was completed, the acetonitrile was removed by concentration under reduced pressure, and 25 mL of ethyl acetate was added to dissolve, and the same volume was used in turn. water, saturated sodium bicarbonate solution and saturated sodium chloride solution were extracted, the organic phase was dried with...
Embodiment 2
[0032] 1-Cyclopropyl-6-fluoro-7-(4-((5-(3-hydroxyphenyl)-1,3,4-oxadiazol-2-yl)methyl)piperazin-1-yl )-4-oxy-1,4-dihydroquinoline-3-carboxylic acid (IIb) preparation steps:
[0033]
[0034] 8.0 mmol of 3-hydroxybenzoic hydrazide, 9.6 mmol of chloroacetyl chloride, and 30 mL of ethyl acetate were added to a 100 mL flask, and the reaction was stirred at room temperature for 3 hours. After the reaction was completed, filtered and air-dried to obtain the precursor compound. 4.8 mmol of the precursor compound, 9.4 mmol of phosphorus oxychloride, and 20 mL of acetonitrile were added to a 100 mL flask, and the reaction was stirred at 70 °C for 12 hours. After the reaction was completed, the acetonitrile was removed by concentration under reduced pressure, and 25 mL of ethyl acetate was added to dissolve it. water, saturated sodium bicarbonate solution and saturated sodium chloride solution were extracted, the organic phase was dried with anhydrous sodium sulfate, and then spin-dri...
Embodiment 3
[0037] 1-Cyclopropyl-6-fluoro-7-(4-((5-(4-hydroxyphenyl)-1,3,4-oxadiazol-2-yl)methyl)piperazin-1-yl )-4-oxy-1,4-dihydroquinoline-3-carboxylic acid (IIc) preparation steps:
[0038]
[0039] 8.0 mmol of 4-hydroxybenzoic hydrazide, 9.6 mmol of chloroacetyl chloride, and 30 mL of ethyl acetate were added to a 100 mL flask, and the reaction was stirred at room temperature for 3 hours. After the reaction was completed, filtered and air-dried to obtain the precursor compound. 4.8 mmol of the precursor compound, 9.4 mmol of phosphorus oxychloride, and 20 mL of acetonitrile were added to a 100 mL flask, and the reaction was stirred at 70 ° C for 20 hours. After the reaction was completed, the acetonitrile was removed by concentration under reduced pressure, and 25 mL of ethyl acetate was added to dissolve, and the same volume was used in turn. water, saturated sodium bicarbonate solution, saturated sodium chloride solution extraction, after the organic phase was dried with anhydrou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com