Chitosan/Mxene antibacterial composite sponge for hemostasis and preparation method thereof
A technology of composite sponge and chitosan, applied in application, pharmaceutical formulation, surgical adhesive and other directions, can solve the problems of poor flexibility of hemostatic sponge, increase process complexity, and insignificant hemostatic effect, and is beneficial to large-scale industrial production. , Improve flexibility, expand the effect of application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of chitosan / Mxene antibacterial composite sponge for hemostasis, concrete steps are as follows:
[0033] (1) under stirring condition, join the chitosan that molecular weight is 100,000 to be dissolved in the acetic acid solution that volume percent concentration is 0.4%, obtain the chitosan acetic acid solution that chitosan concentration is 5g / L;
[0034] (2) Under stirring conditions, add N-(2-hydroxyethyl)acrylamide (the mass ratio of N-(2-hydroxyethyl)acrylamide to chitosan is 3:10) into chitosan-acetic acid solution and mix Uniformly, a mixed solution is obtained;
[0035] (3) Under stirring conditions, add Ti to the mixed solution 3 C 2 T x , mixed evenly to obtain 0.5g / L Ti 3 C 2 T x Chitosan / N-(2-hydroxyethyl)acrylamide / Ti 3 C 2 T x The mixed solution; the mixed solution is vacuum freeze-dried to obtain the chitosan / Mxene antibacterial composite sponge for hemostasis.
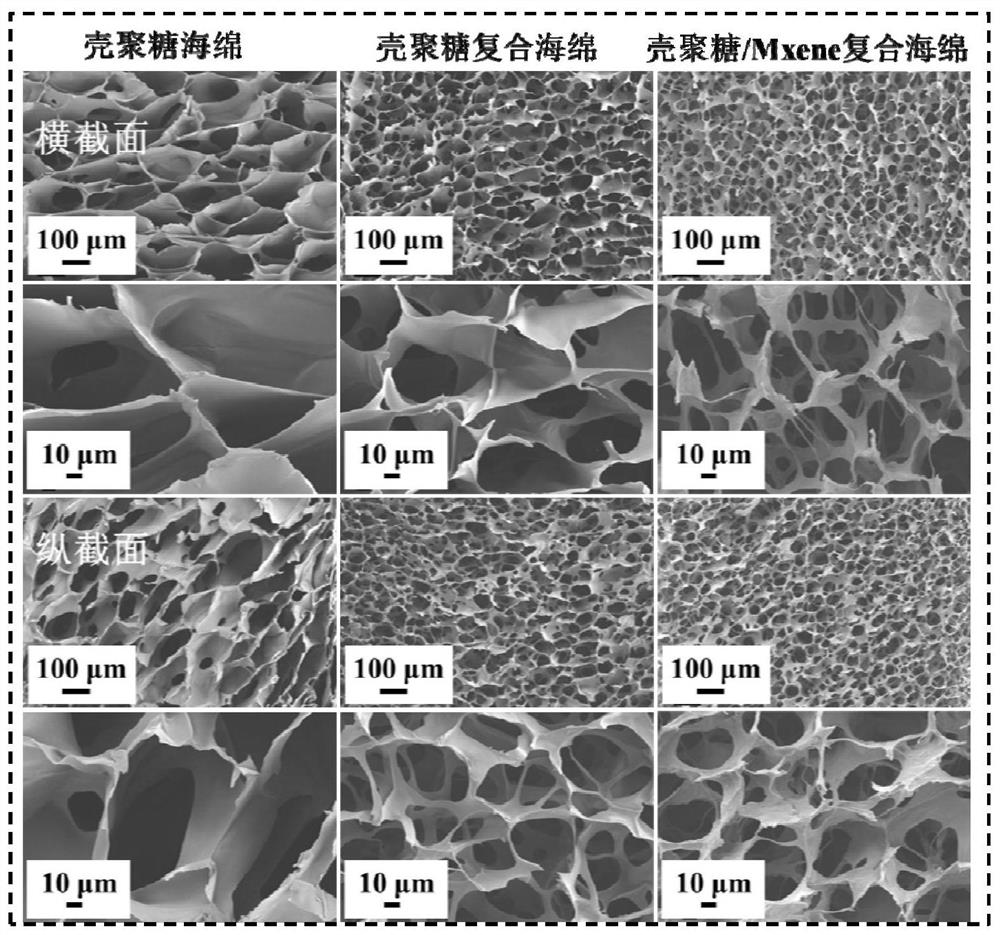
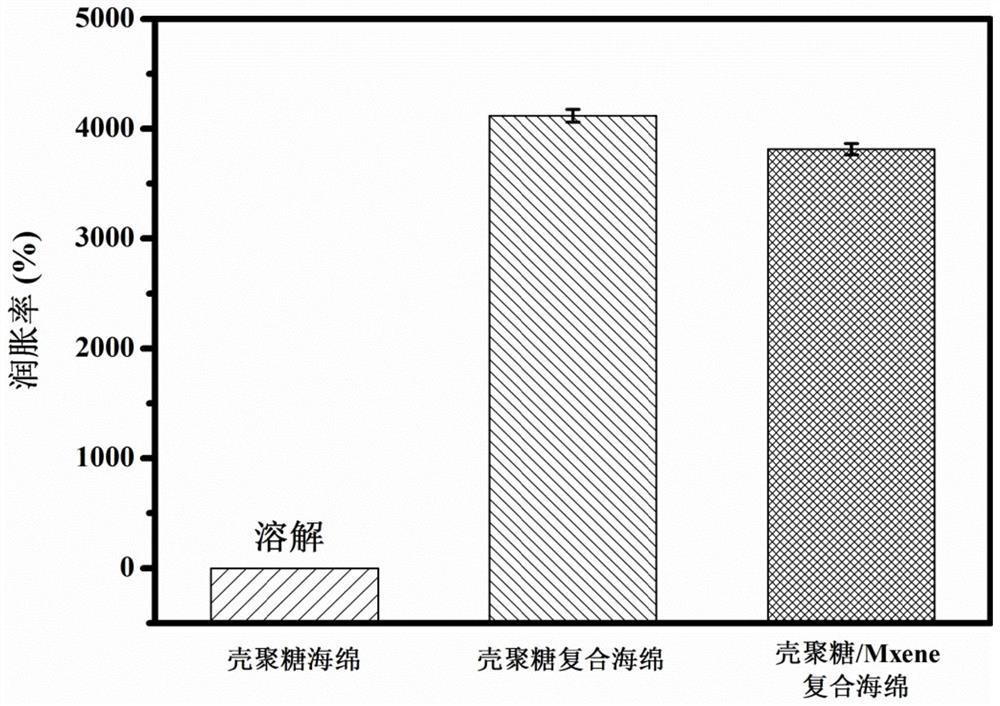
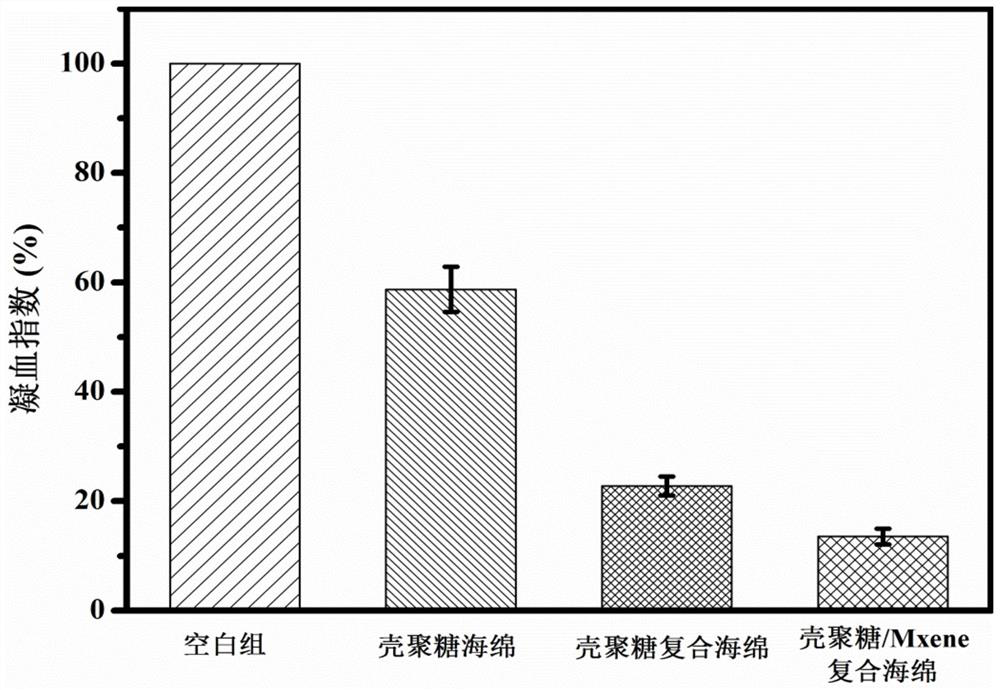
[0036] The chitosan / Mxene antibacterial composite sponge prepared ...
Embodiment 2
[0040] The preparation of chitosan / Mxene antibacterial composite sponge for hemostasis, concrete steps are as follows:
[0041] (1) under stirring condition, molecular weight is that 200,000 chitosans are joined in the acetic acid solution that volume percent concentration is 0.5%, obtain the chitosan acetic acid solution that chitosan concentration is 5g / L;
[0042] (2) Under stirring conditions, add N-(2-hydroxyethyl)acrylamide (mass ratio to chitosan is 1:2) into the chitosan acetic acid solution and mix evenly to obtain a mixed solution;
[0043] (3) Under stirring conditions, add Ti to the mixed solution 3 C 2 T x , mixed evenly to obtain 5g / LTi 3 C 2 T x Chitosan / N-(2-hydroxyethyl)acrylamide / Ti 3 C 2 T x The mixed solution; the mixed solution is vacuum freeze-dried to obtain the chitosan / Mxene antibacterial composite sponge for hemostasis.
[0044] The microscopic appearance data picture of the chitosan / Mxene antibacterial composite sponge prepared by embodiment 2...
Embodiment 3
[0048] The preparation of chitosan / Mxene antibacterial composite sponge for hemostasis, concrete steps are as follows:
[0049] (1) under stirring condition, adding molecular weight is that 250,000 chitosan is dissolved in the acetic acid solution that volume percentage concentration is 0.8%, obtains the chitosan solution that chitosan concentration is 5g / L;
[0050] (2) Under stirring conditions, add N-(2-hydroxyethyl)acrylamide (the mass ratio of N-(2-hydroxyethyl)acrylamide to chitosan is 1:1) into chitosan acetic acid solution and mix Uniformly, a mixed solution is obtained;
[0051] (3) Under stirring conditions, add Ti to the mixed solution 3 C 2 T x , mixed evenly to obtain 20g / LTi 3 C 2 T x Chitosan / N-(2-hydroxyethyl)acrylamide / Ti 3 C 2 T x The mixed solution; the mixed solution is vacuum freeze-dried to obtain the chitosan / Mxene antibacterial composite sponge for hemostasis.
[0052] The chitosan / Mxene antibacterial composite sponge prepared in Example 3 pre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com