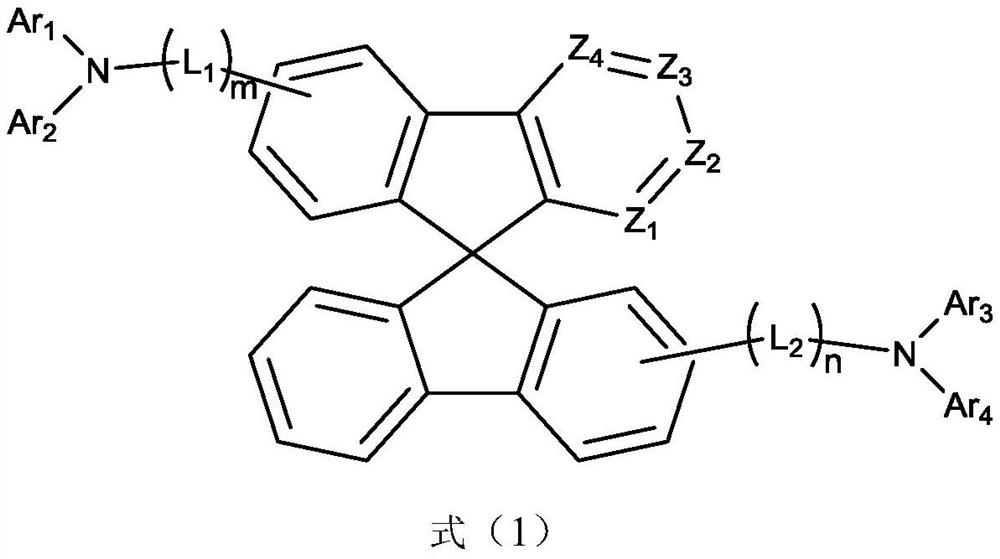
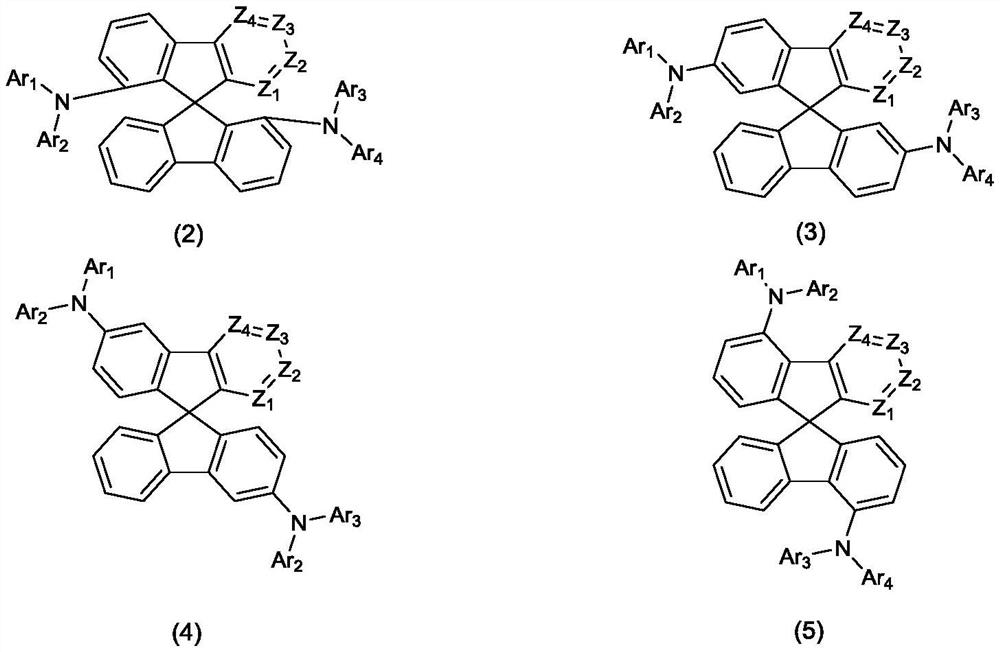
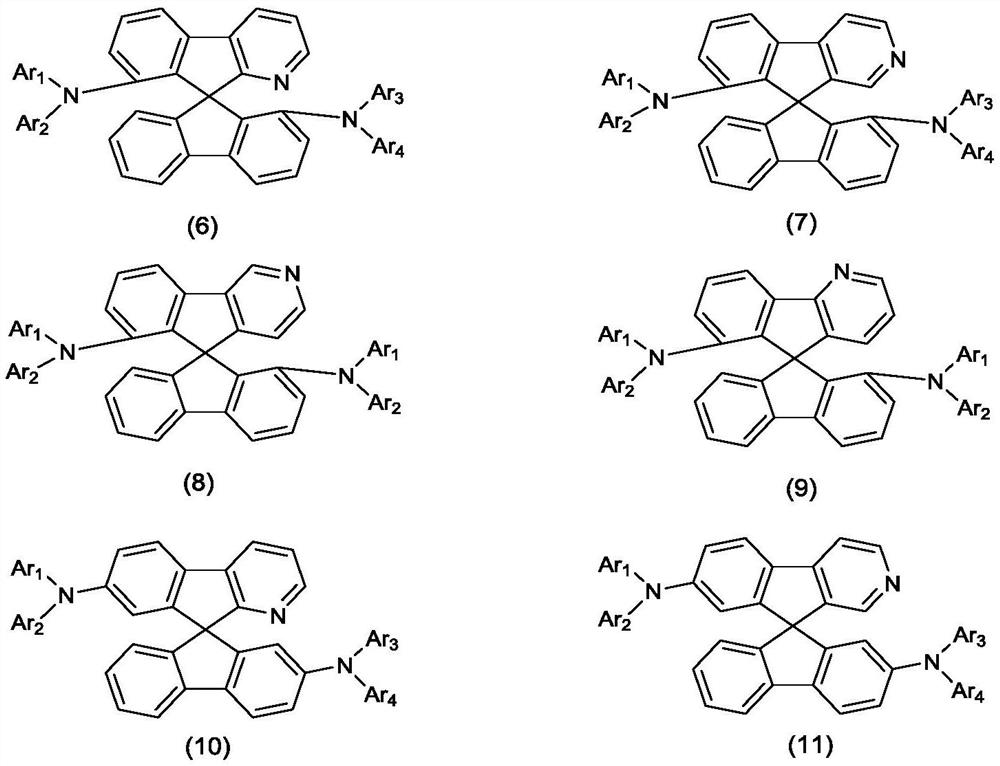
Organic compound with symmetrical iso-substituted aza-spirobifluorene structure and application thereof
An organic compound, azaspiro technology, applied in the field of organic electroluminescent elements, can solve the problems of insufficient carrier transport and thermal stability, improve the glass transition temperature and thermal stability, and is not easy to crystallize Good effect of chemical and film forming
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] The preparation process of the above organic electroluminescent element can be used:
[0054] Method 1, by means of sublimation method to apply one or more layers, where the degree of vacuum in the vacuum sublimation device should usually be less than 10 -5 Mbar, preferably less than 10 -6 The primary pressure of mbar is deposited, in order to ensure the life of the device, it is also preferred to be less than 10 -7 The primary pressure of millibar.
[0055] Second, one or more layers are applied by OVPD (organic gas deposition) method or by means of carrier gas sublimation, thereby at 10 -5 Application of materials under pressure of mbar to 1 bar. Specific embodiments of this method are OVJP (organic vapor print) method, and the material is applied directly through the nozzle.
[0056] Method 3, by spin coating, or by means of any desired printing method such as screen printing, flexographic printing, nozzle printing or lithographic printing, particularly preferably LITI (...
preparation Embodiment 1-1
[0094]
[0095] Synthetic steps:
[0096] The experimental unit was sufficiently dried, and D1 22.8 g (40 mmol) and N-phenylnaphthalene-1-amine 9.6 g (44 mmol) were added to 500 ml of four-mouth flask, and then 300 ml of dry and degassed toluene were added. 5.8 g (60 mmol) tert-butanol sodium, 0.75 g (0.8 mmol) PD 2 (DBA) 3 The catalyst was raised to 80 ° C, slowly added 2 ml of a concentration of 10% to a tetra-t-butylphosphine / toluene solution, and then heated to 100-105 ° C, and the reaction was 6 h. At the end of the reaction, cooled to room temperature, diluted with toluene, pad 200-300 silica gel filtration, the filtrate was evaporated to the solvent, resulting in a crude product, and the crude toluene and n-hexane mixed solvent were recrystallized, resulting in 23.1 g Object 1-1, yield It was 71%, and the purity of HPLC was 99.71%, and finally the vacuum sublimated twice, HPLC purity was 99.99%; MS [M + H] + = 758.42.
preparation Embodiment 1-2
[0098]
[0099] The compounds 1-4 were prepared using the same synthesis of compound 1-1, and the N-phenyl-[1,1'-biphenyl] -3-amine is used in the use of N-phenyl-[1,1'-biphenyl-1-amine. . The yield was 67%, HPLC purity was 99.56%, and finally the vacuum sublimation was purified twice, HPLC purity was 99.99%; MS [M + H] + = 784.42.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com