IL-2 receptor complex and its preparation method and application
A technology of IL-2, 1. IL-2, applied in the field of genetic engineering, can solve the problems of limitations, inability to directly form high-affinity IL-2 receptor complexes, etc., achieve high-affinity activity, and realize large-scale production and preparation , Highly reproducible effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1 Construction of recombinant expression vector
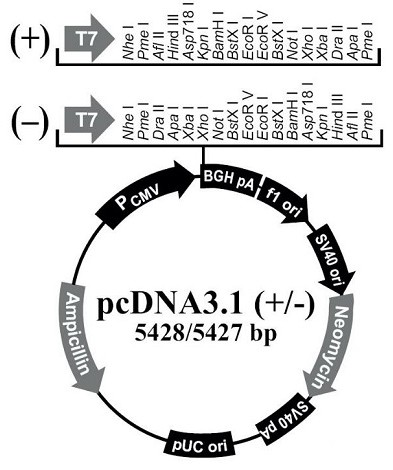
[0058] Construction of the fusion protein I recombinant expression vector pHEK-B1: a polynucleotide encoding an amino acid sequence as shown in SEQ ID NO: 1 was synthesized by Shanghai Bioengineering Co., Ltd. (the amino acid sequence encoding the protein is shown in SEQ ID NO: 2), and in A restriction endonuclease BamH Ⅰ cutting site and a Kozak sequence were added to the 5' end; a stop codon TAA and a restriction endonuclease Xho Ⅰ cutting site were added to the 3' end. The synthesized product was double-digested with restriction endonucleases BamH Ⅰ and Xho Ⅰ, then subjected to agarose gel electrophoresis, and the target fragment was recovered by cutting the gel. The target fragments were respectively combined with pcDNA3.1(+) (Invitrogen Company, catalog number: V790-20) ( figure 2 ) connection to construct the recombinant eukaryotic expression vector pHEK-B1, transform Escherichia coli E.coli DH5α, culture...
Embodiment 2
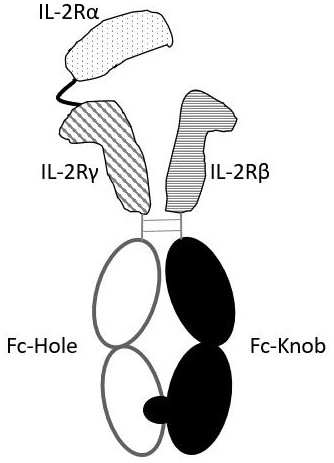
[0060] Example 2 Recombinant expression vector transfected HEK293 cells and expressed IL-2R αβγ heterotrimeric protein
[0061] Using PEI transfection reagent, the constructed recombinant plasmids pHEK-B1 and pHEK-AG1 were transfected into HEK293 cells at a mass ratio of 1:1 for recombinant expression of heterotrimers. The day before transfection, HEK293 cells were divided into 1 × 10 6 / mL subcultured at 37°C. On the day of transfection, count and adjust the cell density to 1.8×10 6 -2.2×10 6 / ml, the activity rate is over 95%.
[0062] Calculate the amount of each component in the transfection complex according to the cell density: the corresponding relationship between the plasmid dose and the number of cells is 1×10 6 The cells correspond to 1 ug of plasmid, and the corresponding relationship between the dose of PEI and the dose of plasmid is that the quality of PEI is 3 times the quality of DNA.
[0063] Prepare the transfection complex according to the amount of com...
Embodiment 3
[0064] Example 3 Separation and Purification of IL-2R αβγ Heterotrimeric Protein
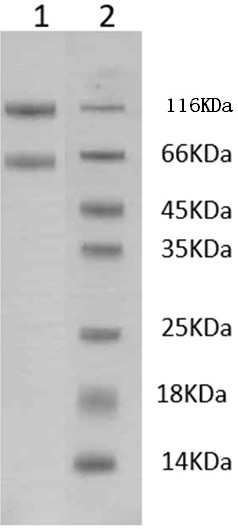
[0065] Use the method of Protein A affinity chromatography to separate and purify the heterotrimeric recombinant protein prepared in Example 2: 4°C, centrifuge at 1000g for 30 min to collect the cell culture supernatant, filter the supernatant and combine with binding buffer beforehand (20mM Tris-HCl, 150mM NaCl, pH 8.0) equilibrated Protein A chromatography column (Stuofan Bioengineering Technology (Guangzhou) Co., Ltd., catalog number: 17543803), and then washed with elution buffer (100 mM glycine, 150 mM NaCl , pH3.5) to elute the target protein, add 1 / 40 volume of neutralization buffer (2M Tris-HCl, pH8.0) to neutralize after collecting the protein eluate, and perform SDS-PAGE ( image 3 ) and HPLC analysis ( Figure 4 ). According to the results of SDS-PAGE, the purified two bands are located around 66KDa and 116KDa respectively, in line with the theoretical molecular weight of protein (I...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com