Lipase mutant D163F with improved catalytic activity and application thereof
一种催化活性提高、D163F的技术,应用在酶工程领域,能够解决不能满足工业需求等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Preparation of embodiment 1 lipase mutant
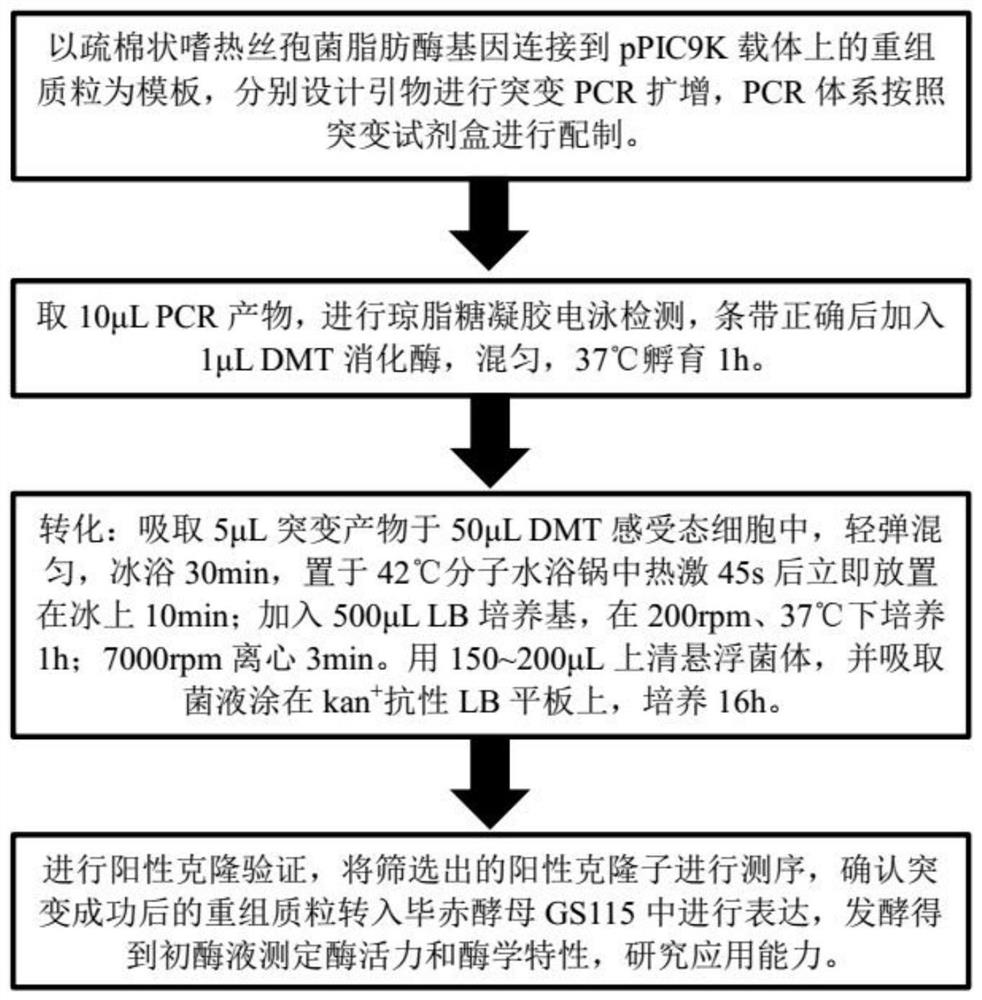
[0049] Such as figure 1 As shown, using the site-directed mutagenesis kit, the pPIC9K-TLL recombinant plasmid was used as a template, and the following primers were used for site-directed mutagenesis amplification. After the amplification is completed, take 10 μL of the PCR product for agarose gel electrophoresis detection. After verifying that the band size is correct, add 1 μL of DMT enzyme to the PCR product, mix well, and digest at 37°C for 1 hour. Then transform: add 3 μL of digested product to 50 μL of DMT competent cells, ice bath for 30 minutes, then heat shock in 42 °C molecular water bath for 45 seconds, ice bath for 2 min, add 500 μL LB medium to the product, at 37 °C, 180 rpm Incubate in a shaker for 1 hour, and finally take 200 μL of bacterial liquid and apply it to kan + Resistant LB dishes were cultured overnight in a 37°C incubator. On the second day, a single colony on the plate was randomly selected for po...
Embodiment 2
[0058] Embodiment 2 lipase mutant enzyme activity and the mensuration of enzymatic properties
[0059] 1. Determination of lipase mutant enzyme activity
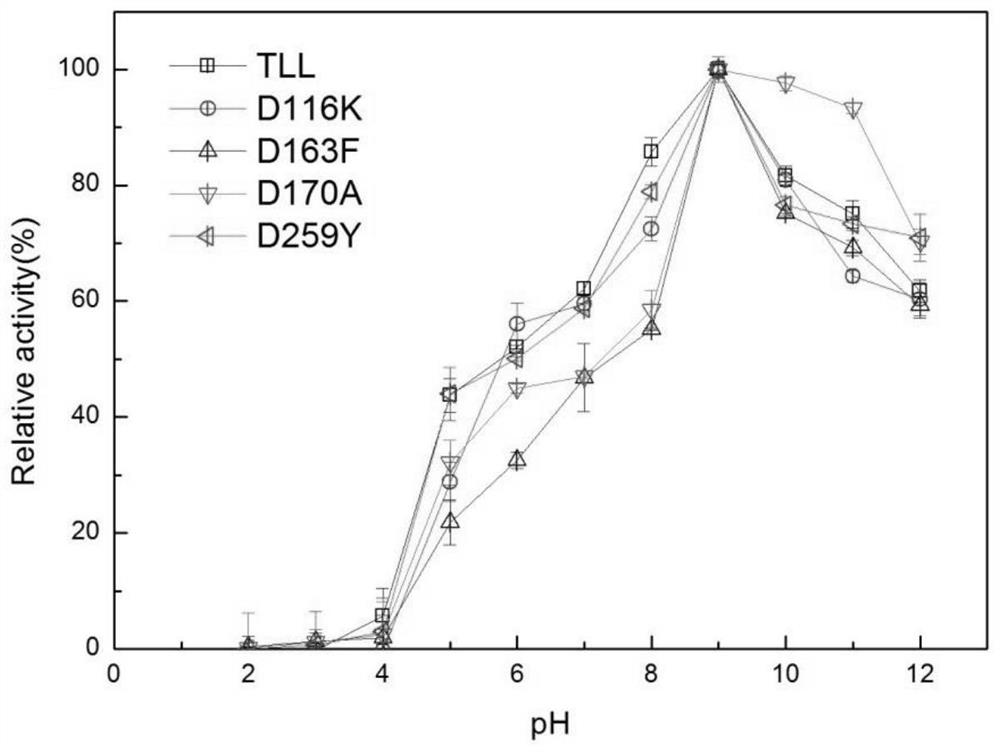
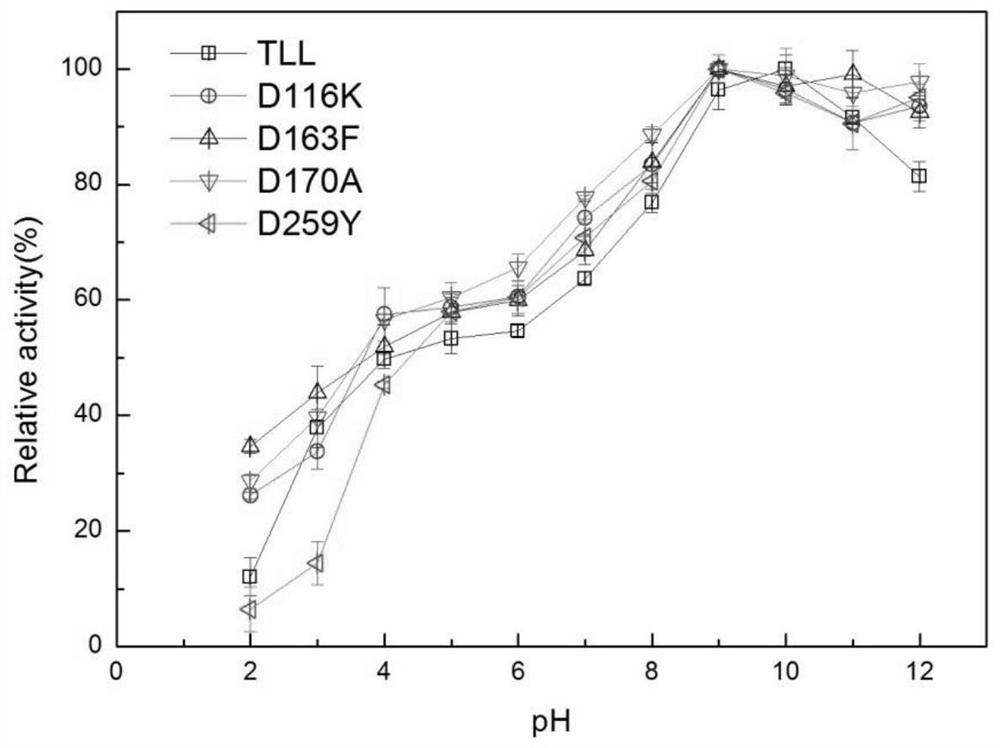
[0060] The enzyme activity unit is defined as: under certain conditions, the amount of enzyme required to hydrolyze the substrate p-NP per minute to generate 1 μmoL of p-nitrophenol is expressed as an enzyme activity unit expressed in U. p-Nitrophenol method: Pipette 420 μL of 50 mM Tris-HCl buffer solution with a pH of 9.0 into a centrifuge tube, then add 30 μL of 10 mM substrate p-NP, mix well, preheat at 37°C for 2 minutes, and then add the diluted 50 μL of enzyme solution was reacted for 5 minutes, 50 μL of 10% SDS was added to terminate the reaction, and finally 500 μL of 0.5 M sodium carbonate was added for color development, and the OD value was measured with a microplate reader at a wavelength of 405 nm. The results of the enzyme activity determination of the mutants are shown in Table 1: the enzyme activities of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com