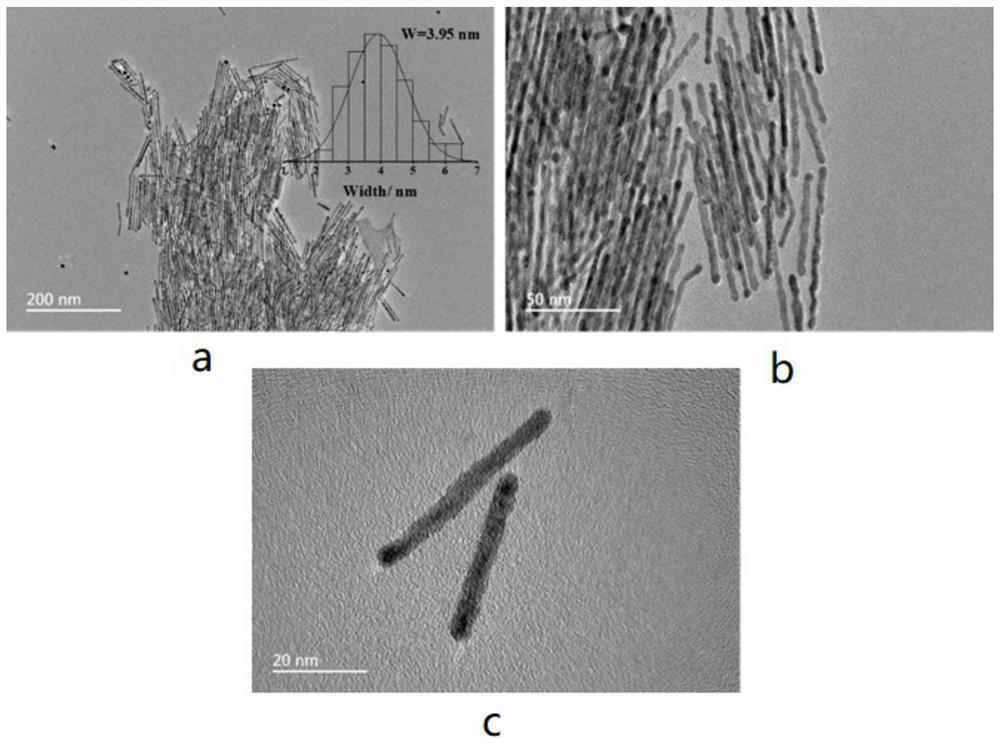
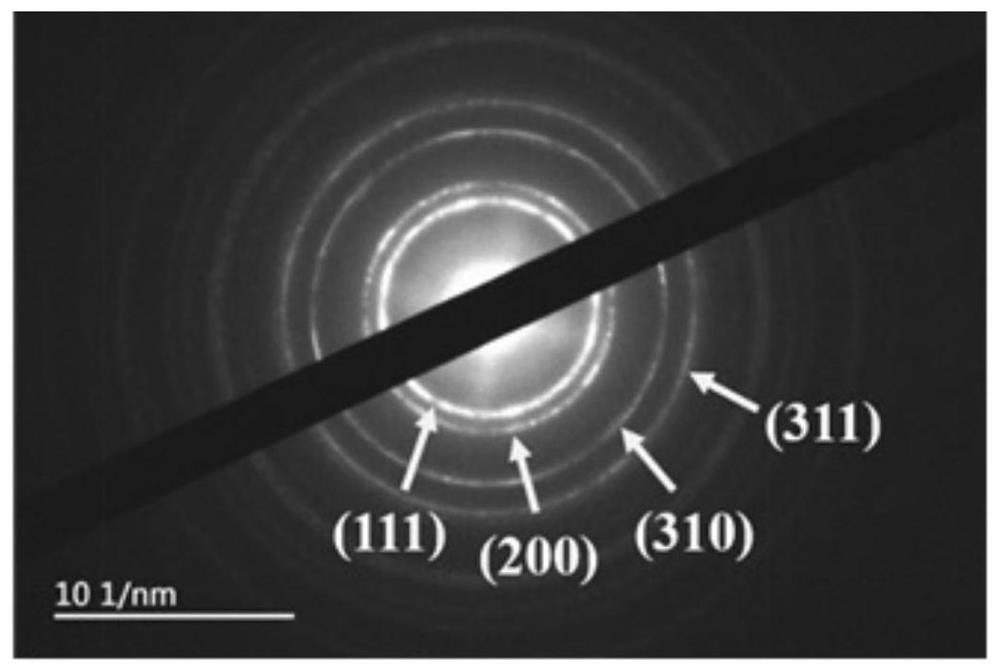
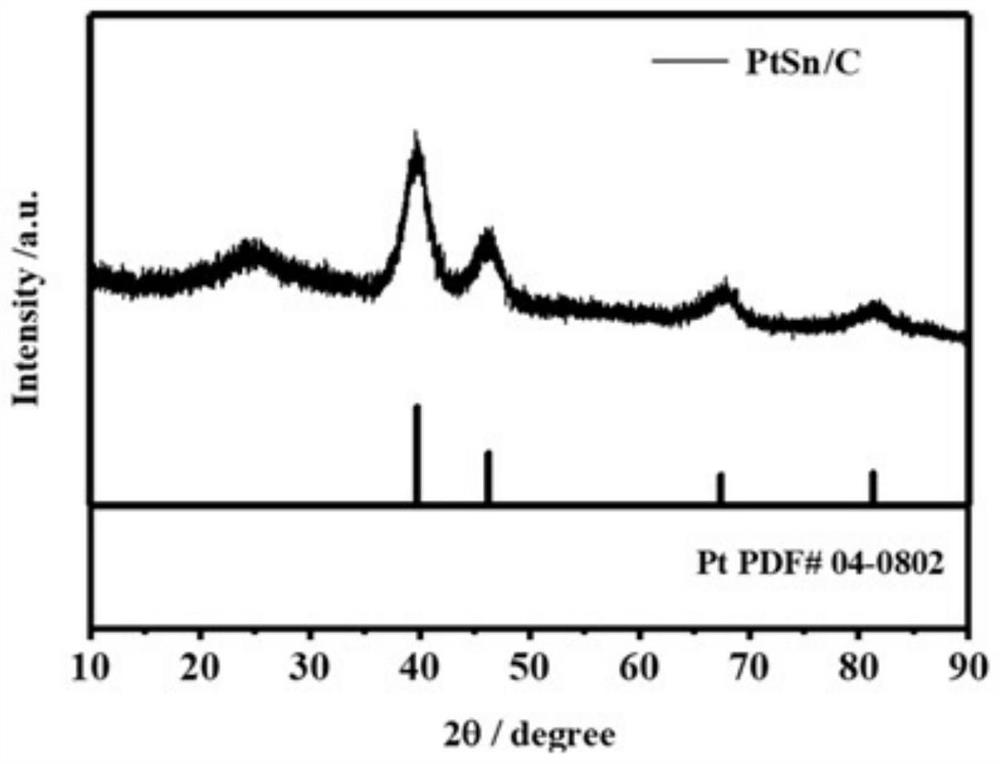
A bimetallic ptsn/c catalyst for high activity fuel cells and its preparation and application
A fuel cell and bimetal technology, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of poor operation durability and high cost, improve catalytic stability, simple and safe preparation process, and facilitate electron transfer. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] A preparation method of a bimetallic PtSn / C catalyst for a high-activity fuel cell, the preparation method specifically comprises the following steps:
[0032] (a) Pt(acac) 2 and CTAB were added to oleylamine and ultrasonically stirred, then W(CO) was added 6 and SnCl 2 ·2H 2 O forms a reaction system and conducts a heating reaction to obtain a bimetallic PtSn material;
[0033] (b) After the reaction system is lowered to room temperature, the bimetallic PtSn material obtained in step (a) is washed and then loaded onto the activated carbon, and then post-treated to obtain a bimetallic PtSn / C catalyst.
[0034] Wherein, in step (a), Pt(acac) 2 , CTAB, W(CO) 6 , SnCl 2 ·2H 2The addition ratio of O and oleylamine is (14-17) mg: (55-65) mg: (5-10) mg: (13-16) mg: (4-8) ml, and the temperature of the heating reaction is 190 -210℃, the heating reaction time is 2-4h. In step (b), adopt the mixed solution containing ethanol and cyclohexane to wash, in the mixed solutio...
Embodiment 1
[0038] A bimetallic PtSn / C catalyst for a high-activity fuel cell is prepared by the following preparation method:
[0039] 15mg of Pt(acac) 2 (Purity: 97%, Shanghai Aladdin Biochemical Technology Co., Ltd., the same below) and 60mg of CTAB were added to 5ml of oleylamine, and the reagent was uniformly dispersed by ultrasonic stirring (the power of the ultrasonic wave was the parameter value commonly used in the laboratory, namely Yes, to achieve the effect of uniform dispersion, the same below), heat the reaction system to 200 ° C, add 8 mg W(CO) 6 and 15mg SnCl 2 ·2H 2 O and kept for 3 hours, the bimetallic PtSn material was obtained by the reaction. After cooling to room temperature, the bimetallic PtSn material was centrifuged and washed 3 times with a mixed solution of ethanol and cyclohexane with a volume ratio of 1:1, and then the bimetallic PtSn material was dispersed in the Loaded in an ethanol solution containing Vulcan XC-72R activated carbon, ultrasonically stir...
Embodiment 2
[0044] A bimetallic PtSn / C catalyst for a high-activity fuel cell is prepared by the following preparation method:
[0045] 14mg of Pt(acac) 2 Add 55mg of CTAB to 4ml of oleylamine, ultrasonic stirring to make the reagent dispersed uniformly, heat the reaction system to 190℃, add 5mg W(CO) 6 and 13mg SnCl 2 ·2H 2 0 and kept for 4 h, the bimetallic PtSn material was obtained by the reaction. After cooling to room temperature, the bimetallic PtSn material was centrifuged and washed three times with a mixed solution of ethanol and cyclohexane with a volume ratio of 0.8:1, and then the bimetallic PtSn material was dispersed in the Loaded in an ethanol solution containing Vulcan XC-72R activated carbon, ultrasonically stirred for 2 h, after loading, suction filtration was performed in sequence, dried in a vacuum drying oven at 50 ° C for 14 hours, and the dried samples were ground for use to obtain bimetallic PtSn / C catalyst, in which the PtSn material has a one-dimensional str...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com