Methods for preparing cholesterol, and derivatives and analogs thereof
An ethanol and compound technology, applied in the field of medicinal chemistry, can solve the problems of limited source of raw materials, low yield and high difficulty in purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
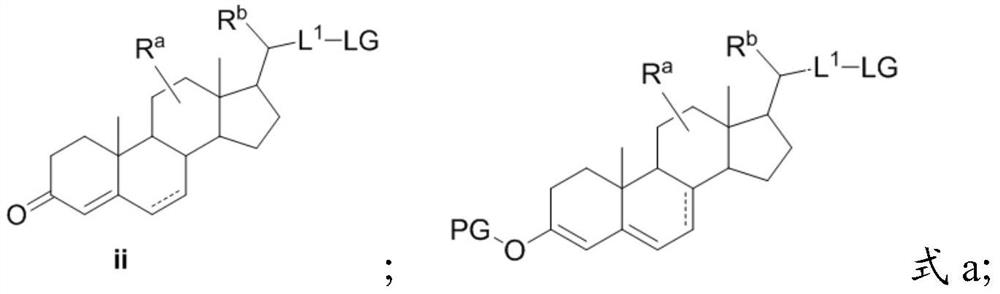
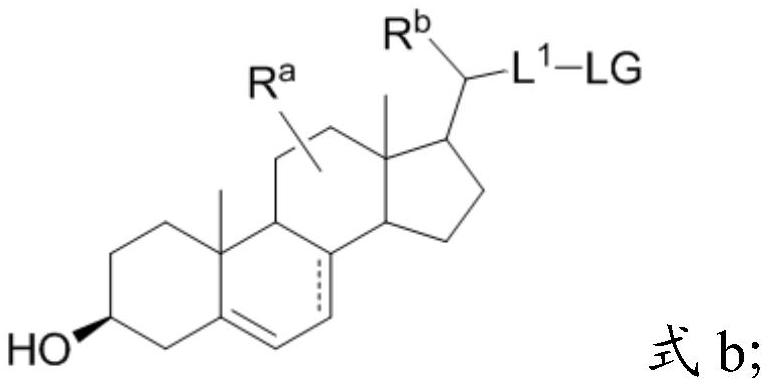
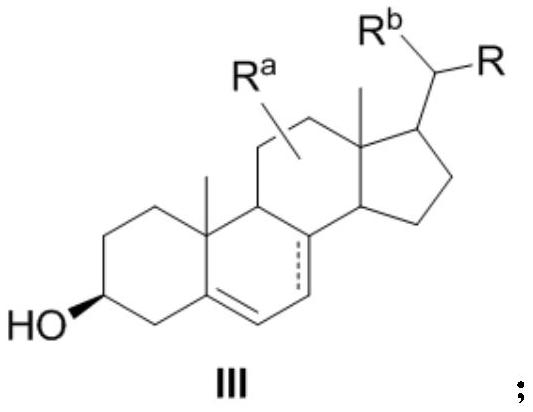
[0075] Preparation method of the present invention
[0076]A first aspect of the present invention provides a method for preparing a compound of formula III, comprising step (A) and step (B), and step (B) comprises step (B-1) or (B-2); wherein
[0077] Step (A):
[0078]
[0079] Step (B):
[0080] Step (B-1):
[0081]
[0082] Step (B-2):
[0083]
[0084] in, Simultaneously represents a single bond in formula I, formula II, and formula III, or "" simultaneously represents a double bond in formula I, formula II, and formula III, or Represent single bond or double bond in formula I, represent double bond in formula II and formula III;
[0085] R a , R b each independently selected from H, -OH, C 1-3 alkyl;
[0086] PG is a hydroxyl protecting group, preferably C 1-8 Silyl, acetyl, trifluoroacetyl, or optionally replaced by one or more C 1-8 Alkyl substituted benzoyl;
[0087] R is L 1 -LG or L 1 -R 1 ;
[0088] L 1 does not exist, or is C 1-8 alk...
Embodiment 1
[0312] Example 1: Preparation of Cholesterol by Compound 1
[0313]
[0314] Step (1) Preparation of Intermediate 2A
[0315]Add 200 g of compound 1, 10 g of DMAP, 200 mL of triethylamine and 1000 mL of DCM into the reaction flask at room temperature, replace with nitrogen, and stir until the solution is clear. Heat to reflux. A solution of 150 g of p-toluenesulfonyl chloride in DCM (400 mL) was added dropwise to the system, and the dropwise addition was completed in about 30 minutes, and the reflux reaction was continued for 1-2 hours. The reaction was monitored by TLC until the starting material disappeared. After the reaction was completed, the temperature of the system was lowered to 10° C., 80 mL of 50% methanol aqueous solution was added dropwise to quench the reaction, and then 600 mL of water was added to separate the layers, and the organic layer was washed with water. Concentrate under reduced pressure to remove most of the solvent, add an appropriate amount of...
Embodiment 2
[0323] Embodiment 2: Preparation of 7-dehydrocholesterol by compound 1
[0324]
[0325] Step (1) see embodiment 1.
[0326] Step (2) intermediate 2a
[0327] At room temperature, add intermediate 2A (100g), anhydrous methanol 500mL, PTS (5g), trimethyl orthoacetate (80mL) into the reaction flask under stirring, keep at 30°C, the reaction is completed in about 3h, TLC monitoring, raw material reaction After completion, add 400mL of acetone, 70mL of water, 80g of chloranil, and slowly raise the temperature to about 40°C under stirring to react, monitor by TLC, the reaction is completed in about 3-4h, pour the reaction system into 1000mL of water to precipitate solid, filter, solid Heat to 50°C with 400mL chloroform to dissolve, filter while hot, heat the filter cake with 100mL chloroform to dissolve, filter, and combine the organic phases. Add saturated aqueous sodium sulfite solution (containing 50 g of sodium sulfite) to the organic phase and stir for 1 h, let stand to s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com